Abstract
Phospholipids are the crucial components of biological membranes and signal transduction. Among different tissues, flower phospholipids are one of the least characterized features of plant lipidome. Here, we report that floral reproductive organs of Arabidopsis thaliana contain high levels of phosphatidic acid (PA), a known lipid second messenger. By using floral homeotic mutants enriched with specific floral organs, lipidomics study showed increased levels of PA species in ap3-3 mutant with enriched pistils. Accompanied gene expression study for 7 diacylglycerol kinases and 11 PA phosphatases revealed distinct floral organ specificity, suggesting an active phosphorylation/dephosphorylation between PA and diacylglycerol in flowers. Our results suggest that PA is a major phospholipid class in floral reproductive organs of A. thaliana.
Phospholipids are the fundamental components of biological membranes, which are categorized into different classes according to the structure of polar head group. For example, in leaves of higher plants, phosphatidylcholine (PC), phosphatidylethanolamine (PE) and phosphatidylglycerol (PG) are major phospholipid classes which have choline, ethanolamine or glycerol as the head group, respectively, while phosphatidic acid (PA) or phosphatidylinositol (PI) are minor phospholipid classes whose head groups are phosphate or inositol, respectively. These minor phospholipid classes cannot be overlooked as they may function as a signal transducer; PA serves as a second messenger in the signaling cascade and PI is phosphorylated to be phosphatidylinositol 4,5-bisphosphate or other phosphorylated derivatives for phosphoinositide signaling.Citation1,2 The composition of membrane lipids differs among different organelles, and even to a greater extent in different tissues. Since these polar head groups have physically diverse property, altered membrane lipid composition may affect surrounding cellular environment and thus molecular processes.
Flowers are the crucial organs for successful propagation of species that show dramatic morphological alteration during reproductive processes. However, the profile of membrane glycerolipids has only recently been started to explore. In previous studies using floral organs of Petunia hybrida, it was shown that membrane lipid biosynthesis is stimulated during flower development,Citation3 and lipid composition is significantly different among floral organs.Citation4 In Arabidopsis thaliana whose flowers are too tiny to perform analytical biochemistry, sensitive lipidomics technology has shown distinct lipid profiles in mature flowers.Citation5 Moreover, dynamic profile of membrane lipids during flower development was revealed by using a transgenic system that synchronizes flower development to facilitate massive sample harvesting.Citation6 Furthermore, an attempt to analyze floral-organ specific lipid profile of Arabidopsis was made by using floral homeotic mutants, ap3-3 and ag-1.Citation7 The advantage of using homeotic mutants is to facilitate sampling of flower enriched with specific floral organs; ap3-3 mutant affects APETALA3 and converts petals to sepals and stamens to pistils.Citation8 The ag-1 mutant, defective in AGAMOUS, produces a large number of petals but not stamens or pistils.Citation9 Since these floral organs are enriched with specific floral organs, floral organ-specific lipid profile can be discussed by comparing the lipid profiles of these mutants.
An interesting feature of flower lipids is high PA level in stamens and pistils, which was previously found in Petunia flower.Citation4 In these reproductive organs, PA level is greater than 10 mol% of total membrane lipids; whereas, it is less than 3 mol% in petals or leaves. In pistils, the PA level is as high as that of PC, a predominant membrane lipid class, which makes PA as one of major phospholipid classes in pistils. Since PA is recognized as a second messenger in plants, this remarkably high level of PA in the reproductive organs of Petunia flower might have some functional relevance. However, this feature has not been reported yet in other plant species such as A. thaliana, leaving a possibility that the high PA level is specially observed in Petunia. The previous lipidomic analysis during flower development did not reveal floral organ-specific profiles of PA.Citation6 Here, we utilized floral homeotic mutants of A. thaliana to analyze PA in floral reproductive organs.
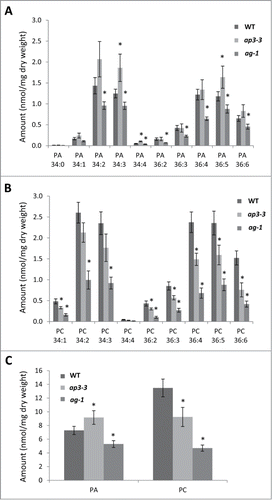
We analyzed PA profiles in mature flowers of ap3-3, ag-1 and the wild type by LC-MS/MS. To avoid artificial production of PA during the lipid extraction due to phospholipase D activity, floral samples were treated with hot isopropanol as described in the legend of . As shown in , levels of primary PA molecular species in flowers were considerably higher in ap3-3 than in ag-1. In particular, ap3-3 increased most of C34 species but only one C36 species as compared to the wild type. To compare the abundance in PA with the major phospholipid class, we quantified levels of primary PC molecular species by LC-MS/MS (). Compared to the flowers of wild type, levels of primary PC molecular species in flowers of ap3-3 were significantly lower, and those of ag-1 were the lowest among 3 samples. Furthermore, we compared total PA and PC levels among flowers of wild type, ap3-3 and ag-1 (). The highest PA level was observed in ap3-3 flowers, whereas the highest PC level was seen in wild-type flowers. Because PA level was found as high as PC level in ap3-3 flowers, this indicates that PA is enriched significantly in ap3-3 flowers. We suggest that reproductive organs of A. thaliana contain high levels of PA.
Figure 1. Phosphatidic acid (PA) profiles of the flowers of ap3-3 and ag-1 compared to the wild type analyzed by triple quadrupole LC-MS/MS. (A) Levels of major PA molecular species. (B) Levels of major PC molecular species. (C) Levels of total PA and PC. Data are mean ±SD of 4 biological replicates. Asterisks indicate significance (p < 0.01) from wild type. Mature flowers of wild type or homeotic mutants (ap3-3 and ag-1) were harvested, immediately frozen in liquid nitrogen, and kept at −80°C until lipid extraction. Prior to lipid extraction, frozen tissues were incubated in hot (75°C) isopropanol containing 0.05% (v/v) butylated hydroxytoluene (Cat. No. B1378, Sigma-Aldrich, St. Louis, MO) for 15 min to inhibit phospholipase D activity for PA production. PA and phosphatidylcholine (PC) were analyzed as follows; the dried lipid fractions extracted from plant tissues were dissolved in 200 μl chloroform/methanol 1/1 (v/v). Samples were then transferred (10 μl) into MS vials and mixed with an equal volume of a 0.2 μg/ml solution of internal standards dimyristoylphosphatidic acid (PA 14:0/14:0, 0.32 nmol/ml) and dimyristoylphosphatidylcholine (PC 14:0/14:0, 0.30 nmol/ml) in solvent A (95% acetonitrile + 5% 10mM ammonium acetate). LC-MS/MS analyses were performed using an Agilent 1200 series HPLC-Chip system connected to an Agilent 6490 Triple quadrupole. Hydrophilic interaction chromatography (HILIC) separation was undertaken using a HILIC-chip containing Amide-80 stationary phase (5 µm particle size, 80Å pore size), including a 160 nl trapping column and a 5 µm × 150 mm analytical column (Agilent Technologies Corp., Santa Clara, CA). Separation was achieved using a 19 min gradient of solvent A (95% acetonitrile + 5% 10 mM ammonium acetate) and solvent B (50% acetonitrile + 50% 10 mM ammonium acetate). Gradient was as follows: 100% A for 1.5 min, linear gradient to 90% B over 11.5 min, 90% B for 0.5 min, to 10% B over 0.1 min, 100% A until end of run (total runtime 19 min). Samples were injected (1 μl) through the enrichment column at 4 µl/min. The valve was switched 1.5 min after injection to place the enrichment column in line with the analytical column at a flow rate of 400 nl/min. The mass spectrometer was operated using the following parameters: gas temperature 185°C, gas flow 12 l/min, capillary voltage 1580 V. Multiple reaction monitoring (MRM) transitions (PA to fatty acid fragments) were monitored with a collision energy of 33 V and a fragmentor value of 380 V. Quantification was done by normalizing the area under the curve (AUC) of the chromatographic peak of each MRM transition to the AUC of the internal standard peaks.
To investigate to what extent this lipid profile is associated with the transcriptional changes of relevant lipid metabolic genes, we performed qRT-PCR analysis using mature flowers of wild type, ap3-3 and ag-1. As the central metabolites of plant glycerolipid metabolism, PA and sn-1,2-diacylglycerol (DAG) can be produced by multiple pathways involving a number of enzymes. Here, we focus on the direct inter-conversion between PA and DAG; PA is dephosphorylated by PA phosphatase (PAP) to be DAG.Citation10 In turn, DAG is phosphorylated by DAG kinase (DGK) to produce PA.Citation11 First, we referred to a published microarray data of the Affymetrix ATH1 Arabidopsis Genome Array data for polar glycerolipid biosynthetic genes expressed in different floral organs.Citation12 This was then compared with our qRT-PCR data.
Arabidopsis has 7 DGK isoforms, DGK1-DGK7.Citation13 As shown in , the published microarray data revealed that DGK1, DGK3, and DGK5 are the primarily expressed isoforms in pistils, stamens, and petals, whereas DGK4 is highly expressed only in stamens. In addition, the expression of DGK5 is slightly higher in stamens and petals than pistils. Our analysis with qRT-PCR showed that the expression of DGK7 was the highest among DGK isoforms, and DGK4 and DGK6 were the lowest (). While it is in agreement with the result of microarray data that DGK1, DGK3 and DGK5 are primarily expressed isoforms and DGK4 and DGK6 are the least expressed isoforms (), it is inconsistent with the microarray data that expression level of DGK7 is the highest among 7 isoforms and DGK2 is as high as DGK1 in expression level. DGK4 and DGK5 showed significant reduction in ap3-3 compared to the wild type, suggesting that they are highly expressed in stamens. DGK4 was lower in ap3-3 and ag-1, suggesting that DGK4 is preferentially expressed in stamens. DGK5 showed reduced expression only in ap3-3, suggesting that stamens are the major site for DGK5 expression.
Figure 2. Gene expression analysis for DGKs and PAPs in the flowers of ap3-3, ag-1 and wild type of Arabidopsis thaliana by quantitative reverse transcription-PCR (qRT-PCR) in comparison with published microarray data of wild-type floral organs. (A, B) Transcript levels of DGKs (A) and PAPs (B) in different floral organs of Arabidopsis thaliana by published microarray data (Affymetrix ATH1 Arabidopsis Genome Arrary).Citation12 (C, D) Relative gene expression of 7 DGKs (C) and 11 PAPs (D) analyzed by quantitative qRT-PCR in the flowers of wild type, ap3-3, and ag-1. Data are mean ±SD of 6 replicates including 3 technical replicates of 2 biological replicates. Asterisks indicate significance (p < 0.01) from wild type. Total RNA was extracted from the samples using RNeasy plant mini kit (Qiagen), followed by reverse-transcription to cDNA by SuperScript III reverse-transcription kit (Invitrogen) as described previously.Citation7 qRT-PCR was performed with specific primers designed for each target genesCitation6 using 7500 Real Time PCR System (Life Technologies, Carlsbad, CA). Actin was used as a reference,Citation6 and primer specificity was examined previously.Citation6
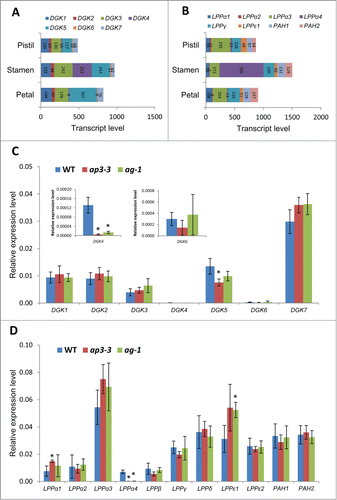
Two types of PAPs are known in A. thaliana:Citation10 membrane-bound lipid phosphate phosphatase (LPP)Citation14,15 and soluble phosphatidate phosphohydrolase (PAH1 and PAH2).Citation16 Arabidopsis has 9 homologs of LPP, known as LPP1-LPP4/LPPα1-LPPα4, LPPβ, LPPγ, LPPδ, LPPϵ1 and LPPϵ2.Citation14,15 Not all of those PAP genes are available for the expression data on Affymetrix ATH1. As shown in , microarray data showed that LPPα3 is the most dominantly expressed isoform, which is followed by LPPγ, PAH1 and PAH2. Here, our qRT-PCR results confirmed the highest expression level of LPPα3 (). Moreover, LPPγ, LPPδ, LPPϵ1 and LPPϵ2 were found to be expressed as high as PAH1 and PAH2. Of the 11 genes shown, only 3 (LPPα1, LPPα4, LPPϵ1) were found to have significantly different expression levels in floral homeotic mutants. LPPα1 is known to be strongly expressed in leaves compared to flowers.Citation15 Our qRT-PCR analysis showed a notably increased expression levels of LPPα1 in ap3-3 as compared to the wild type (), suggesting that the expression of LPPα1 is enriched in pistils although microarray data showed relatively higher expression level of LPPα1 in petals. shows that LPPϵ1 was expressed higher in ag-1 than in wild type, suggesting that LPPϵ1 is highly expressed in petals.
The gene expression analysis on DGKs and PAPs in floral organs revealed primarily expressed isoforms in flowers as well as those showing floral organ specificity. Although the transcript levels of these genes do not appear to show clear association with PA levels in reproductive organs, it was shown that distinct set of genes are stimulated in different floral organs that suggests active inter-conversion between PA and DAG via DGKs and PAPs. To date, involvement of these gene functions in flower development is still obscure due to the lack of gene knockout study of most of these genes with altered flower phenotype. A recently reported organ fusion phenotype in the flowers of pah1 pah2 mutant is intriguing.Citation6 Since PAH1 and PAH2 are involved in the major PAP activityCitation16,17 and PA level showed transient increase at early stages of flower development,Citation6 it is possible that PA in reproductive organs may have critical role in flower development that are regulated by complex interplay among these PA metabolic genes. In conclusion, our study showed that PA is a major phospholipid class in reproductive organs of Arabidopsis thaliana. Further studies are anticipated to unravel the role of PA during plant reproductive processes.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Kazue Kanehara for critical reading of the manuscript.
Funding
This research was supported by the core budget provided by IPMB, Academia Sinica. Y.N is supported by EMBO Young Investigator Program.
References
- Testerink C, Munnik T. Molecular, cellular, and physiological responses to phosphatidic acid formation in plants. J Exp Botany 2011; 62:2349-61; PMID:21430291; http://dx.doi.org/10.1093/jxb/err079
- Boss WF, Im YJ. Phosphoinositide Signaling. Annu Rev Plant Biol 2012; 63:409-29; PMID:22404474; http://dx.doi.org/10.1146/annurev-arplant-042110-103840
- Nakamura Y, Arimitsu H, Yamaryo Y, Awai K, Masuda T, Shimada H, Takamiya K-I, Ohta H. Digalactosyldiacylglycerol is a major glycolipid in floral organs of Petunia hybrida. Lipids 2003; 38:1107-12; PMID:14669976; http://dx.doi.org/10.1007/s11745-006-1166-x
- Nakamura Y, Ohta H. The diacylglycerol forming pathways differ among floral organs of Petunia hybrida. FEBS Letters 2007; 581:5475-9; PMID:17983603; http://dx.doi.org/10.1016/j.febslet.2007.10.053
- Devaiah SP, Roth MR, Baughman E, Li M, Tamura P, Jeannotte R, Welti R, Wang X. Quantitative profiling of polar glycerolipid species from organs of wild-type Arabidopsis and a PHOSPHOLIPASE Dα1 knockout mutant. Phytochemistry 2006; 67:1907-24; PMID:16843506; http://dx.doi.org/10.1016/j.phytochem.2006.06.005
- Nakamura Y, Teo NZW, Shui G, Chua CHL, Cheong W-F, Parameswaran S, Koizumi R, Ohta H, Wenk MR, Ito T. Transcriptomic and lipidomic profiles of glycerolipids during Arabidopsis flower development. New Phytologist 2014; 203:310-22; PMID:24684726; http://dx.doi.org/10.1111/nph.12774
- Nakamura Y, Liu Y-c, Lin Y-C. Floral glycerolipid profiles in homeotic mutants of Arabidopsis thaliana. Biochem Biophys Res Commun 2014; 450:1272-5; PMID:24984150; http://dx.doi.org/10.1016/j.bbrc.2014.06.115
- Jack T, Brockman LL, Meyerowitz EM. The homeotic gene APETALA3 of Arabidopsis thaliana encodes a MADS box and is expressed in petals and stamens. Cell 1992; 68:683-97; PMID:1346756; http://dx.doi.org/10.1016/0092-8674(92)90144-2
- Yanofsky MF, Ma H, Bowman JL, Drews GN, Feldmann KA, Meyerowitz EM. The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature 1990; 346:35-9; PMID:1973265; http://dx.doi.org/10.1038/346035a0
- Nakamura Y, Shimojima M, Ohta H, Shimojima K. Chapter 13 Biosynthesis and Function of Monogalactosyldiacylglycerol (MGDG), the Signature Lipid of Chloroplasts. In: Rebeiz C, Benning C, Bohnert H, Daniell H, Hoober JK, Lichtenthaler H, Portis A, Tripathy B, eds. The Chloroplast: Springer Netherlands, 2010:185-202
- Arisz SA, Testerink C, Munnik T. Plant PA signaling via diacylglycerol kinase. Biochim Biophys Acta 2009; 1791:869-75; PMID:19394438; http://dx.doi.org/10.1016/j.bbalip.2009.04.006
- Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Scholkopf B, Weigel D, Lohmann JU. A gene expression map of Arabidopsis thaliana development. Nat Genet 2005; 37:501-06; PMID:15806101; http://dx.doi.org/10.1038/ng1543
- Gómez-Merino FC, Brearley CA, Ornatowska M, Abdel-Haliem MEF, Zanor M-I, Mueller-Roeber B. AtDGK2, a novel diacylglycerol kinase from Arabidopsis thaliana, phosphorylates 1-stearoyl-2-arachidonoyl-sn-glycerol and 1,2-dioleoyl-sn-glycerol and exhibits cold-inducible gene expression. J Biol Chem 2004; 279:8230-41; PMID:14665624; http://dx.doi.org/10.1074/jbc.M312187200
- Nakamura Y, Tsuchiya M, Ohta H. Plastidic phosphatidic acid phosphatases identified in a distinct subfamily of lipid phosphate phosphatases with prokaryotic origin. J Biol Chem 2007; 282:29013-21; PMID:17652095; http://dx.doi.org/10.1074/jbc.M704385200
- Pierrugues O, Brutesco C, Oshiro J, Gouy M, Deveaux Y, Carman GM, Thuriaux P, Kazmaier M. Lipid phosphate phosphatases in Arabidopsis : regulation of the atlpp1 gene in response to stress. J Biol Chem 2001; 276:20300-8; PMID:11278556; http://dx.doi.org/10.1074/jbc.M009726200
- Nakamura Y, Koizumi R, Shui G, Shimojima M, Wenk MR, Ito T, Ohta H. Arabidopsis lipins mediate eukaryotic pathway of lipid metabolism and cope critically with phosphate starvation. Proc Natl Acad Sci 2009; 106:20978-83; PMID:19923426; http://dx.doi.org/10.1073/pnas.0907173106
- Eastmond PJ, Quettier A-L, Kroon JTM, Craddock C, Adams N, Slabas AR. Phosphatidic acid phosphohydrolase1 and 2 regulate phospholipid synthesis at the endoplasmic reticulum in Arabidopsis. Plant Cell 2010; 22:2796-811; PMID:20699392; http://dx.doi.org/10.1105/tpc.109.071423
