ABSTRACT
The circadian system induces rhythmic variation in a suite of biochemical and physiological processes that serve to optimise plant growth in diel cycles. To be of greatest utility, these rhythmic behaviors are coordinated with regular environmental changes such as the rising and setting of the sun. Photoreceptors, along with metabolites produced during photosynthesis, act to synchronise the internal timing mechanism with lighting cues. We have recently shown that phototropins help maintain robust rhythms of photosynthetic operating efficiency (ϕPSII or Fq′/Fm′) under blue light, although rhythmic accumulation of morning-phased circadian transcripts in the nucleus was unaffected. Here we report that evening-phased nuclear clock transcripts were also unaffected. We also observe that rhythms of nuclear clock transcript accumulation are maintained in phototropin mutant plants under a fluctuating lighting regime that induced a loss of Fq′/Fm′ rhythms.
Monitoring circadian rhythms in planta
While nuclear rhythms of gene expression are routinely measured in planta using luciferase reporter lines, circadian rhythms in the chloroplast can be documented by monitoring light emitted from endogenous chlorophylls following a period of illumination. Delayed Fluorescence (DF) methods monitor light emitted from chlorophyll immediately after extinguishing ambient illumination from growth lightsCitation1 whereas comparison of chlorophyll a fluorescence (CaF) before and immediately after the application of a saturating light pulse allows the operating efficiency of photosystem II to be examined (φPSII or Fq′/Fm′,Citation2-4). Variation in DF or Fq′/Fm′ over time represent 2 methods that can be used to monitor circadian rhythms in the chloroplast.
Light inputs into the circadian system
To be of greatest utility the circadian system is responsive to daily and seasonal variations in photoperiod.Citation5,6 Changes in ambient light and temperature signal into a biological network of interconnected feedback loops.Citation5 Most work has focused upon transcription/translation feedback loops in the nucleus, but recently oscillations in protein oxidation have also been identified that continue in the absence of nuclear rhythms in certain species and tissue types.Citation7,8
Each of the identified photoreceptor families acts to either transmit information into the central circadian oscillator or modulates a circadian output.Citation9 Phytochromes, cryptochromes, and UV-B RESISTANCE8 (UVR8), have been shown to accelerate nuclear clock pace in response to red, blue or UV-B signals respectively,Citation10-12 while the role of the ZTL family in the post-translational regulation of certain circadian components in response to blue light has been well documented.Citation13,14 Both distinct and converging signaling pathways initiated by these photoreceptors act on the nuclear clock although the precise mechanisms involved have yet to be elucidated in many cases.
The phototropin family of blue photoreceptors are atypical in that they have not been ascribed a role within the nuclear circadian system.Citation4,15 We have recently shown that phototropins help to maintain robust rhythms of Fq′/Fm′ under dim blue light, without altering rhythms in the nucleus.Citation4 Here we examine the role of phototropins within the nuclear circadian system in greater detail and confirm that rhythmic transcript accumulation in the nucleus does not appear to be altered in plants lacking both phototropin1 (phot1) and phot2.
Phototropins do not alter expression levels of evening components within the circadian system
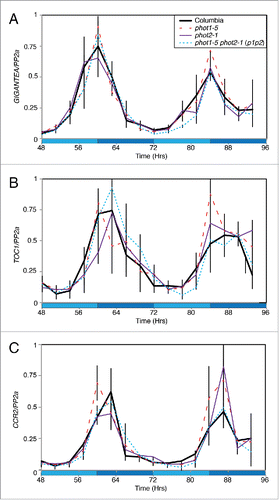
Our recent work used qRT-PCR to demonstrate that accumulation of circadian transcripts was not altered in phot1-5 phot2-1 (p1p2) seedlings but our initial analysis was restricted to morning-phased transcripts.Citation4 To expand our analysis, we examined the accumulation of selected evening-phased transcripts under constant blue light (). As for morning-phased genes, we observed no significant difference in GIGANTEA, TIMING OF CAB1 EXPRESSION1 (TOC1), or COLD, CIRCADIAN RHYTHM AND RNA BINDING2 (CCR2) transcript accumulation in phot1-5, phot2-1 or p1p2 double mutants compared to a wild type control (). Initial analysis of phase and period of these rhythmic transcripts was completed using the JTK_CYCLE algorithm,Citation16 although interpretation of these data are limited by the resolution and length of the qRT-PCR time course. This analysis indicated there was no difference in the phase or period in the rhythms of GIGANTEA transcript accumulation but minor differences were observed in relation to TOC1 and CCR2 transcripts. A modest 1.5 hour phase delay in TOC1 rhythms were detected in phot1-5 and p1p2 seedlings that was not present in phot2-1 while a longer 27-hour period in TOC1 rhythms was returned for phot2-1 and p1p2 lines (compared to 24 hours in wild type and phot1-5 seedlings). Peak CCR2 transcript accumulation was also delayed by 1.5 hours, but only in phot1-5 and phot2-1 seedlings. Instead, CCR2 transcripts may cycle with a longer period in p1p2 lines (27hrs in p1p2 compared to 24hrs in wild type and the phot1-5 and phot2-1 single mutants). Although this analysis may indicate a minimal role for phototropins in the maintenance of rhythmicity of TOC1 and CCR2 (but not GIGANTEA) this proposition will need to be clarified through the use of extended qRT-PCR time courses or via luciferase reporter lines in phototropin mutant backgrounds.
Figure 1. Accumulation of circadian clock-regulated transcripts under constant blue light. Transcript accumulation in wild type (Columbia, solid black), phot1-5 (dashed red), phot2-1 (purple) and phot1-5 phot2-1 (p1p2, dotted blue) mutants was compared using qRT-PCR. Levels of GIGANTEA (A), TOC1 (B), and CCR2 (C) mRNA were assessed. Plants were entrained to 12:12 LD cycles for 12 d on ½ MS media before being moved to constant conditions with 20 μmol m−2 s−1 blue light. Data for each transcript were compared with an internal control (PP2a) before being normalized to the peak of wild-type accumulation. Data are the average of 3 biological replicates, error bars show standard error of the mean. Dark blue shading indicates subjective night.
The introduction of hourly dark intervals does not impair rhythmicity of the nuclear circadian clock in p1p2 seedlings
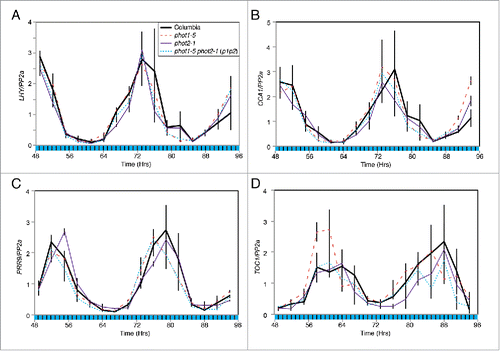
Inclusion of an hourly dark interval into the illumination protocol during CaF imaging induced a reduction in amplitude of Fq′/Fm′ rhythms under 50 µmol m−2 s−1 blue light in p1p2 plantsCitation4 and so we investigated whether these conditions precipitated the loss of nuclear rhythms in these lines (). As under cB, we found that transcript accumulation of LATE ELONGATED HYPOCOTYL (LHY), CIRCADIAN CLOCK ASSOCIATED1 (CCA1), PSEUDORESPONSE REGULATOR9 (PRR9), and TOC1, was unaltered in these conditions of fluctuating blue light (). Such data suggest that phototropins act to maintain robust circadian oscillations of PSII operating efficiency downstream of the central nuclear oscillator and reinforce the notion that phototropins have a minimal role within the nuclear circadian system.
Figure 2. Accumulation of circadian clock-regulated transcripts under fluctuating blue light. Transcript accumulation in wild type (Columbia, solid black), phot1-5 (dashed red), phot2-1 (purple) and phot1-5 phot2-1 (p1p2, dotted blue) mutants was compared using qRT-PCR. Levels of LHY (A), CCA1 (B), PRR9 (C) and TOC1 (D) mRNA were assessed. Plants were entrained to 12:12 LD cycles for 12 d on ½ MS media before being moved to 50 μmol m−2 s−1 blue light interspersed with 10 minute dark intervals every hour. Data for each transcript were compared with an internal control (PP2a) before being normalized to the peak of wild-type accumulation. Data are the average of 3 biological replicates, error bars show standard error of the mean. Black bars indicate periods of darkness during harvesting schedule.
Defining the role of phototropins within the Arabidopsis circadian system
Phototropins are plasma-membrane localized, light-activated kinases that are re-localized to the cytosol, chloroplast outer membrane and golgi apparatus upon illumination with blue light.Citation17-19 Although a nuclear localization of phot2 has been reported as a consequence of overexpressionCitation17 examination of transgenic lines expressing phot2 fused to GFP and a nuclear localization signal (P2G-NLS) revealed that P2G-NLS is less active than phot2 lacking an NLS.Citation17 Indeed, subsequent analysis revealed that a substantial proportion of P2G-NLS is retained at the plasma membrane (in addition to a nuclear population).Citation17 It therefore remains plausible that the observed loss of activity in P2G-NLS lines is a consequence of phot2 sequestration within the nucleus. Such data, in combination with our qRT-PCR assays suggest that phototropins act to amplify Fq′/Fm′ oscillations independently of the nuclear transcription/translation circadian clock.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the Leverhulme Trust (ECF-2012– 358), The Royal Society (grant no. RG130746), The Oppenheimer Memorial Trust (PhD studentship to S.L.), the Gen Foundation, and the University of Essex. The authors would like to thank Prof. John Christie (University of Glasgow, UK) and Prof Stacey Harmer (UC Davis, USA) for the generous gift of seeds used in this study.
References
- Gould PD, Diaz P, Hogben C, Kusakina J, Salem R, Hartwell J, Hall A. Delayed fluorescence as a universal tool for the measurement of circadian rhythms in higher plants. Plant J 2009; 58:893-901; PMID:19638147; http://dx.doi.org/10.1111/j.1365-313X.2009.03819.x
- Baker NR. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Ann Rev Plant Biol 2008; 59:89-113; http://dx.doi.org/10.1146/annurev.arplant.59.032607.092759
- Rascher U, Hütt MT, Siebke K, Osmond B, Beck F, Lüttge U. Spatiotemporal variation of metabolism in a plant circadian rhythm: The biological clock as an assembly of coupled individual oscillators. PNAS 2001; 98:11801-5; PMID:11573013; http://dx.doi.org/10.1073/pnas.191169598
- Litthauer S, Battle MW, Lawson T, Jones MA. Phototropins maintain robust circadian oscillation of PSII operating efficiency under blue light. Plant J 2015; 83:1034-45; PMID:26215041; http://dx.doi.org/10.1111/tpj.12947
- Hsu PY, Harmer SL. Wheels within wheels: The plant circadian system. Tr Plant Sci 2014; 19:240-9; http://dx.doi.org/10.1016/j.tplants.2013.11.007
- Song YH, Shim JS, Kinmonth-Schultz HA, Imaizumi T. Photoperiodic flowering: time measurement mechanisms in leaves. Ann Rev Plant Biol 2015; 66:441-64; http://dx.doi.org/10.1146/annurev-arplant-043014-115555
- O'Neill JS, Van Ooijen G, Dixon LE, Troein C, Corellou F, Bouget F-Y, Reddy AB, Millar AJ. Circadian rhythms persist without transcription in a eukaryote. Nature 2011; 469:554-8; PMID:21270895; http://dx.doi.org/10.1038/nature09654
- Edgar RS, Green EW, Zhao Y, Van Ooijen G, Olmedo M, Qin X, Xu Y, Pan M, Valekunja UK, Feeney KA, et al. Peroxire-doxins are conserved markers of circadian rhythms. Nature 2012; 485:459-64; PMID:22622569; http://dx.doi.org/10.1038/nature11088
- Millar AJ. A suite of photoreceptors entrains the plant circadian clock. J Biol Rhythm 2003; 18:217-26; http://dx.doi.org/10.1177/0748730403018003004
- Devlin P, Kay S. Cryptochromes are required for phytochrome signaling to the circadian clock but not for rhythmicity. Plant Cell 2000; 12:2499-510; PMID:11148293; http://dx.doi.org/10.1105/tpc.12.12.2499
- Somers D, Devlin P, Kay S. Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science 1998; 282:1488-90; PMID:9822379; http://dx.doi.org/10.1126/science.282.5393.1488
- Fehér B, Kozma-Bognár L, Kevei E, Hajdu A, Binkert M, Davis SJ, Schäfer E, Ulm R, Nagy F. Functional interaction of the circadian clock and UV RESISTANCE LOCUS8-controlled UV-B signaling pathways in Arabidopsis thaliana. Plant J 2011; 67:37-48; http://dx.doi.org/10.1111/j.1365-313X.2011.04573.x
- Baudry A, Ito S, Song YH, Strait AA, Kiba T, Lu S, Henriques R, Pruneda-Paz JL, Chua NH, Tobin EM, et al. F-Box proteins FKF1 and LKP2 act in concert with ZEITLUPE to control Arabidopsis clock progression. Plant Cell 2010; 22:606-22; PMID:20354196; http://dx.doi.org/10.1105/tpc.109.072843
- Más P, Kim W-Y, Somers DE, Kay SA. Targeted degradation of TOC1 by ZTL modulates circadian function in Arabidopsis thaliana. Nature 2003; 426:567-70; http://dx.doi.org/10.1038/nature02163
- Devlin PF, Kay SA. Circadian photoperception. Ann Rev Phys 2001; 63:677-94; http://dx.doi.org/10.1146/annurev.physiol.63.1.677
- Hughes ME, Hogenesch JB, Kornacker K. JTK_CYCLE: An efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. J Biol Rhythm 2010; 25:372-80; http://dx.doi.org/10.1177/0748730410379711
- Kong S-G, Suetsugu N, Kikuchi S, Nakai M, Nagatani A, Wada M. Both phototropin 1 and 2 localize on the chloroplast outer membrane with distinct localization activity. Plant Cell Phys 2013; 54:80-92; http://dx.doi.org/10.1093/pcp/pcs151
- Kong S-G, Suzuki T, Tamura K, Mochizuki N, Hara-Nishimura I, Nagatani A. Blue light-induced association of phototropin 2 with the golgi apparatus. Plant J 2006; 45:994-1005; PMID:16507089; http://dx.doi.org/10.1111/j.1365-313X.2006.02667.x
- Sakamoto K, Briggs WR. Cellular and subcellular localization of phototropin 1. Plant Cell 2002; 14:1723-35; PMID:12172018; http://dx.doi.org/10.1105/tpc.003293
