ABSTRACT
In bacteria a second messenger, guanosine 5′-diphosphate 3′-diphosphate (ppGpp), synthesized upon nutrient starvation, controls many gene expressions and enzyme activities, which is necessary for growth under changeable environments. Recent studies have shown that ppGpp synthase and hydrolase are also conserved in eukaryotes, although their functions are not well understood. We recently showed that ppGpp-overaccumulation in Arabidopsis chloroplasts results in robust growth under nutrient-limited conditions, demonstrating that the bacterial-like stringent response at least functions in plastids. To test if ppGpp also functions in the cytosol, we constructed the transgenic Arabidopsis expressing Bacillus subtilis ppGpp synthase gene yjbM. Upon induction of the gene, the mutant synthesizes ∼10–20-fold higher levels of ppGpp, and its fresh weight was reduced to ˜80% that of the wild type. These results indicate that cytosolic ppGpp negatively regulates plant growth and development.
Stringent response is one of the most important regulatory systems for bacteria to survive under fluctuating conditions. The response is mediated by the unusual nucleotide, guanosine 5′-diphosphate 3′-diphosphate (ppGpp), the level of which is maintained by two enzymes, RelA and SpoT, in Escherichia coli.Citation1 These enzymes catalyze ppGpp synthesis by phosphorylating GTP (or GDP) using ATP in response to various environmental signals such as amino-acid starvation; and ion, phosphorus, and carbon limitation. ppGpp controls the transcription and translation of many genes, and regulates several enzyme activities to fit metabolic processes for growth under starved conditions.Citation1
Recent progress in genome sequencing has revealed that RelA/SpoT homologs (RSHs) are conserved in plants and green algae.Citation2-4 Moreover, metazoan SpoT homolog-1 (Mesh1) was identified in human and Drosophila melanogaster.Citation5 Plant RSHs and Drosophila Mesh1 show ppGpp synthase and hydrolase activities, respectively,Citation5-8 suggesting that ppGpp functions in eukaryotic cells. We recently showed that Arabidopsis RSHs, localized in chloroplasts, are important for plant growth and stress responses; ppGpp overaccumulation in chloroplasts results in robust growth under nutrient-deficient conditions.Citation9 This indicates that the stringent response is at least operating in the organelle, which has important roles in the systematic growth of host organisms.
The next question is whether ppGpp could function in the cytosol. In plants, nucleotides synthesized in plastids are transported to the cytosol by several transporters,Citation10,11 suggesting that some ppGpp, synthesized in plastids, could localize in cytosolic fractions. Given that ppGpp competitively binds most GTP-binding sites,Citation12 cytosolic ppGpp, if it exists, should have an impact on many metabolic controls. To check this possibility, we aimed to observe the effects of ppGpp-overaccumulation in the cytosol in plants. Specifically, we constructed a transgenic Arabidopsis expressing Bacillus subtilis ppGpp synthase gene (yjbM) under the control of the estrogen-induced promoter; the resulting line was designated as YJBMox. Because YjbM is a small protein (˜25 kDa) consisting of a single ppGpp synthase domain,Citation13 YjbM expression could induce constitutive ppGpp synthesis in the cytosol.
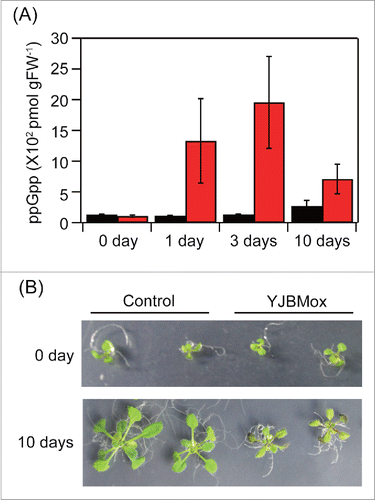
The YJBMox and the control plant, containing the empty vector, were grown under normal 1/2MS mediumCitation9 for 10 days, and then transferred to 1/2MS medium containing 2 μM β-estradiol to induce YjbM overexpression. As shown in , ppGpp levels in YJBMox, but not the control line, were highly increased (∼10–20-fold), with an observed maximum value after 3 days. Given YjbM has no transit peptide, it should localize and produce ppGpp in the cytosol. The observed phenotype of YJBMox was striking; it showed retarded growth on the estrogen-containing plates (). The fresh weight of the YJBMox line was reduced to ∼80% that of the wild type. These results indicated that cytosolic ppGpp negatively regulates plant growth.
Figure 1. ppGpp overaccumulation results in retarded growth of Arabidopsis. The yjbM geneCitation13 was synthesized in vitro with adapting its codon usage for expression in Arabidopsis. The DNA fragment was cloned into the estrogen-inducible expression vector pER8 at its SpeI and XhoI sites.Citation15 The resulting plasmid was introduced into Arabidopsis wild type (ecotype Columbia) by the Agrobacterium transformation method,Citation16 and the obtained mutant was designated as YJBMox. As a control, the empty vector (pER8) was also introduced. (A) Plants grown in MS medium for 10 days were transferred to the MS medium containing 2 μM β-estradiol, and ppGpp levels in each line were quantified after 0, 1, 3, and 10-days. (B) Phenotypes of plants 0 and 10 days after the transfer.
We recently showed that ppGpp overaccumulation in chloroplasts results in better and more robust growth under normal and nutrient-limited conditions, respectively.Citation9 The ppGpp effects on plant growth are the opposite of those observed in the cytosolic ppGpp overaccumulation mutant as reported here, suggesting that cytosolic and plastidial ppGpp levels could regulate plant growth in a different way. Perhaps, in the wild type plant, ppGpp levels are coordinately regulated in each location for optimizing different metabolic processes and gene expressions to adapt the growth rate in response to varying environmental conditions.
It was previously shown that Drosophila Mesh1 mutant exhibited retarded body growth during early developmental stages.Citation5 Given that Mesh1 possesses only a ppGpp-hydrolase domain, ppGpp levels in the mutant cells are expected to be higher than that in the wild type. This suggests that retarded growth of the Mesh1 mutant may result from cytosolic ppGpp overaccumulation, although ppGpp synthase is unknown and there is still no evidence for the existence of ppGpp in animal cells. Clearly, further characterization of plant RSHs and Drosophila Mesh1, coupled with a quantitative analysis of ppGpp,Citation14 should be important for elucidating the exact function and evolutionary traits of the ppGpp-dependent stringent response for metabolic control in eukaryotic cells.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank all Photobiology Lab members for discussion, and L. Kwok (Tokyo Institute of Technology) for critical reading of the manuscript.
Funding
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas from MEXT of Japan (No. 25120709) to S.M.
References
- Cashel M, Gentry DR, Hernandez VJ, Vinella D. The stringent response. In: Escherichia coli and Salmonella; Cell Mol Biol, 2nd edn. 1458-1496, 1996; Neidhardt FC, Curtiss R, III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (Eds.), ASM Press, Washington, USA
- Masuda S. The stringent response in phototrophs. In: Advances in Photosynthesis - Fundamental Aspects. 487-500, 2012; Najafpour M (Ed.), In Tech
- Tozawa Y, Nomura Y. Signalling by the global regulatory molecule ppGpp in bacteria and chloroplasts of land plants. Plant Biol 2011; 13:699-709; PMID:21815973; http://dx.doi.org/10.1111/j.1438-8677.2011.00484.x
- van der Biezen EA, Sun J, Coleman MJ, Bibb MJ, Jones JD. Arabidopsis RelA/SpoT homologs implicate (p)ppGpp in plant signaling. Proc Natl Acad Sci 2000; 97:3747-3752; PMID:10725385; http://dx.doi.org/10.1073/pnas.97.7.3747
- Sun D, Lee G, Lee JH, Kim HY, Rhee HW, Park SY, Kim KJ, Kim Y, Kim BY, Hong JI, et al. A metazoan ortholog of SpoT hydrolyzes ppGpp and functions in starvation responses. Nat Struct Mol Biol 2010; 17:1188-1194; PMID:20818390; http://dx.doi.org/10.1038/nsmb.1906
- Givens RM, Lin MH, Taylor DJ, Mechold U, Berry JO, Hernandez VJ. Inducible expression, enzymatic activity and origin of higher plant homologues of bacterial RelA/SpoT stress proteins in Nicotiana tabacum. J Biol Chem 2004; 279:7495-504; PMID:14660585; http://dx.doi.org/10.1074/jbc.M311573200
- Masuda S, Mizusawa K, Narisawa T, Tozawa Y, Ohta H, Takamiya K. The bacterial stringent response, conserved in chloroplasts, controls plant fertilization. Plant Cell Physiol 2008; 49:135-141; PMID:18178586; http://dx.doi.org/10.1093/pcp/pcm177
- Tozawa Y, Nozawa A, Kanno T, Narisawa T, Masuda S, Kasai K, Nanamiya H. Calcium-activated (p)ppGpp synthetase in chloroplasts of land plants. J Biol Chem 2007; 282:35536-35545; PMID:17938177; http://dx.doi.org/10.1074/jbc.M703820200
- Maekawa M, Honoki R, Ihara Y, Sato R, Oikawa A, Kanno Y, Ohta H, Seo M, Saito K, Masuda S. Impact of the plastidial stringent response in plant growth and stresss responses. Nat Plants 2015; 1:15167, http://dx.doi.org/10.1038/nplants.2015.167
- Neuhaus HE, Wagner R. Solute pores, ion channels and metabolite transporters in the outer and inner envelope membranes of higher plant plastids. Biochim Biophys Acta 2000; 1465:307-323; PMID:10748262; http://dx.doi.org/10.1016/S0005-2736(00)00146-2
- Spetea C, Schoefs B. Solute transporters in plant thylakoid membranes. Commun Integr Biol 2010; 3:122-129; PMID:20585503; http://dx.doi.org/10.4161/cib.3.2.10909
- Kanjee U, Ogata K, Houry WA. Direct binding targets of the stringent response alarmone (p)ppGpp. Mol Microbiol 2012; 85:1029-1043; PMID:22812515; http://dx.doi.org/10.1111/j.1365-2958.2012.08177.x
- Nanamiya H, Kasai K, Nozawa A, Yun CS, Narisawa T, Murakami K, Natori Y, Kawamura F, Tozawa Y. Identification and functional analysis of novel (p)ppGpp synthetase genes in Bacillus subtilis. Mol Microbiol 2008; 67:291-304; PMID:18067544; http://dx.doi.org/10.1111/j.1365-2958.2007.06018.x
- Ihara Y, Ohta H, Masuda S. A highly sensitive quantification method for the accumulation of alarmone ppGpp in Arabidopsis thaliana using UPLC-ESI-qMS/MS. J Plant Res 2015; 128:511-518; PMID:25752614; http://dx.doi.org/10.1007/s10265-015-0711-1
- Zuo J, Niu QW, Chua NH. An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J 2000; 24:265-273; PMID:11069700; http://dx.doi.org/10.1046/j.1365-313x.2000.00868.x
- Bechtold N, Pelletier G. In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol Biol 1998; 82:259-266; PMID:9664431
