ABSTRACT
Cellulose is a cell wall constituent that is essential for plant growth and development, and an important raw material for a range of industrial applications. Cellulose is synthesized at the plasma membrane by massive cellulose synthase (CesA) complexes that track along cortical microtubules in elongating cells of Arabidopsis through the activity of the protein CELLULOSE SYNTHASE INTERACTING1 (CSI1). In a recent study we identified another family of proteins that also are associated with the CesA complex and microtubules, and that we named COMPANIONS OF CELLULOSE SYNTHASE (CC). The CC proteins protect the cellulose synthesising capacity of Arabidopsis seedlings during exposure to adverse environmental conditions by enhancing microtubule dynamics. In this paper we provide cell biology and genetic evidence that the CSI1 and the CC proteins fulfil distinct functions during cellulose synthesis. We also show that the CC proteins are necessary to aid cellulose synthesis when components of the CesA complex are impaired. These data indicate that the CC proteins have a broad role in aiding cellulose synthesis during environmental changes and when core complex components are non-functional.
Plant cell walls are essential structures for directed cell growth, plant stature, cell-cell communication and adherence, and for protection against environmental factors.Citation1,3,17,18 Most plant cell walls are built from carbohydrate-containing polymers, including cellulose, hemicelluloses and pectins, which are interlinked to form a strong but flexible architecture.Citation18 Cellulose is the most abundant biopolymer on Earth, and provides a load-bearing scaffold for other cell wall polymers.Citation12 Cellulose consists of β-1,4-glucan chains that form microfibrils via hydrogen-bonding, and are synthesized by large Cellulose Synthase (CesA) complexes (CSCs) at the plasma membrane.Citation12 The CSCs are made up of a catalytic core that typically consists of a heterotrimeric configuration of the CesA proteins. For example, CesA1, CesA3 and CesA6-related proteins form the catalytic core of the CSC necessary for primary wall cellulose.Citation5,14 The CSCs are thought to be assembled in the Golgi apparatus and then trafficked to the plasma membrane via the trans-Golgi network (TGN;11) and a small CesA-containing compartment referred to as small Cellulose Synthase compartments (smaCCs;Citation8) and/or Microtubule Associated Small CesA compartments (MASCs;Citation4). The delivery of the CesAs to the plasma membrane spatially coincides with cortical microtubules.Citation8 Once inserted into the membrane the CSCs begin to synthesize cellulose, which is extruded into the cell wall. It is believed that the cellulose microfibrils become trapped in the cell wall, and further synthesis thus leads to movement of the CSC through the plasma membrane.Citation12 The direction of the CSC movement is in many cells guided by cortical microtubules via the protein CELLULOSE SYNTHASE INTERACTING 1 (CSI1/POM2), which can bind to both the CesA proteins and microtubules.Citation2,7,9
Apart from the CesA proteins, several components that partake in cellulose synthesis have been identified. These include the CHITINASE-LIKE (CTL) proteins, CTL1 and 2,Citation16 the Glycosylphosphatidylinisotol (GPI) anchored protein COBRA,Citation10,15 and the endoglucanase KORRIGAN.Citation13,19 These proteins can be closely associated with the CSCs, but do not appear to have a central role in the catalytic process of cellulose synthesis. Recently, two new components of the CSC were identified.Citation11 These components interacted with the CesA proteins and moved together with the CSCs during cellulose synthesis. The proteins were therefore named COMPANIONS OF CELLULOSE SYNTHASE (CC) 1 and 2. Lesions in CC1 and CC2 did not display any major phenotypic changes compared to wild-type when grown under normal conditions; however, when grown on media supplemented with either cellulose synthesis inhibitors, microtubule inhibitors or salt, the cc1 cc2 double mutant seedlings displayed stunted growth and cell swelling.Citation11 In vitro and in vivo work revealed that the cytosolic part of the CC proteins could interact with microtubules and promoted microtubule dynamics. The enhanced microtubule dynamics mediated by the CC proteins were, furthermore, necessary to maintain cellulose synthesis during salt stress (Endler at al., 2015). Hence, the CC proteins are important factors for sustained cellulose synthesis during adverse conditions, which is mediated through the re-establishment of the microtubule array.
While both the CSI1/POM2 and the CC proteins can interact with both the CesAs and the microtubules it is not clear if the connection between the CSC and the microtubules is impaired in cc1 cc2 mutants. Such disruption has been shown for the csi1/pom2 mutants, which provided the basis for the notion that the protein promotes a guiding function of the CSC along the cortical microtubules during cellulose synthesis.Citation2,9 To test whether the CC proteins affected microtubule-based guidance of the CSCs we assessed the alignment of the trajectories of moving CSC and the cortical microtubules using a dual-labeled line with YFP-CesA6 and mCHERRY-TUA5 in a cc1 cc2 mutant background under mild salt (100 mM NaCl). To promote germination, seeds were first sown on MS media and after two days seedlings were transferred to MS plates containing 100 mM NaCl. After two further days of growth the dual-labeled lines were imaged by spinning disc confocal microscopy. shows that individual YFP-CesA6 fluorescent foci are predominantly associated with microtubules (; left panel), and that the trajectories of the CSC movements clearly align with the microtubules in time average images (; right panel). We quantified the co-occurrence of the two signals and found identical values for both, cc1 cc2 and wild-type under salt stress (). Thus, the CC proteins do not influence microtubule-based guidance of the CSCs.
Figure 1. (A–B) Association of CesAs and microtubules in cc1 cc2 mutants. (A) Spinning disc confocal microscopy of a hypocotyl cell of a 4-day-old etiolated cc1 cc2 seedling expressing YFP-CesA6 (YFP-C6) and mCHERRY-TUA5 (mCh-TUA5). Seedlings were germinated and grown for 2 days on MS plates, and were then transferred to plates containing 100 mM NaCl. After 2 days growth on the salt-containing media the dual-labeled line were imaged by spinning disc confocal microscopy.Citation11 In single frame images (left panel) the majority of YFP-CesA6 foci co-localize with microtubules. The overlapping signal of YFP-CesA6 and mCh-TUA5 in time averages (150 frames, 5 sec interval) indicating that CESAs move along trajectories of cortical MTs in cc1 cc2 double mutants. Bar = 5 µm. (B) Quantification of co-occurrence of YFP-CesA6 and mCh-TUA5 signals in time average images as those in (A). Both markers show identical values for co-localization in cc1 cc2 and wild-type (p-value = 0.4, Welch's unpaired t-test). (C–D) Genetic interaction of cc1 cc2 with pom2-4 or prc1-1. (C) Five-day-old Col-0, cc1, cc2, cc1 cc2, pom2-4 and cc1 cc2 pom2-4 seedlings grown on MS media (left panel). Bar = 1 mm. Five-week-old Col-0, cc1, cc2, cc1 cc2, pom2-4 and cc1 cc2 pom2-4 plants grown on soil (right panel). Note the reduced hypocotyl length and growth of cc1 cc2 pom2-4 compared to pom2-4, respectively. Bar = 2 cm (D) Five-day-old col-0, cc1, cc2, cc1 cc2, prc1-1 and cc1 cc2 prc1-1 seedlings grown on MS media (left panel). Bar = 1 mm. Five-week-old col-0, cc1, cc2, cc1 cc2, prc1-1 and cc1 cc2 prc1-1 plants grown on soil. Note the reduced hypocotyl elongation and rosette size of cc1 cc2 prc1-1 in comparison to prc1-1, respectively. Bar = 2 cm. (E) Quantification of hypocotyl length of genotypes from C and D; n=30 seedlings. **p ≤ 0.001. Student's t test, error bars are SD.
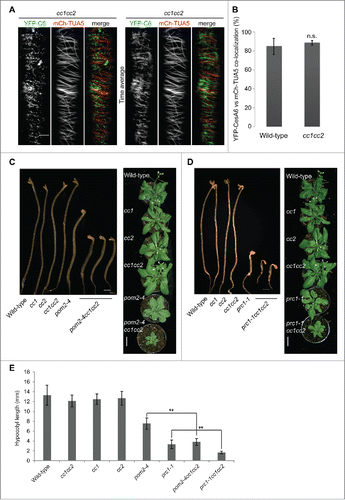
To further investigate whether there are genetic interactions between the CSI1/POM2 and the CC proteins we introgressed the csi1/pom2 mutant allele pom2-4 Citation2 into the cc1 cc2 mutant background. We found that the growth of the homozygous triple mutants was substantially reduced as compared with the cc1 cc2 and pom2-4 parent lines (). The mutant phenotypes appeared more severe then what would be expected by a simple additive effect between the two mutants and can therefore be considered aggravating mutations. It is plausible that the CC proteins could maintain a function that guides the CSCs, or maintains CSC function at the plasma membrane in the absence of microtubule guidance by CSI1. To explore the relationships between the CSC components and the CC proteins further, we also produced homozygous cc1 cc2 prc1-1 triple mutants. prc1-1 holds a null mutation in CesA6 that has a non-essential function in the CSC due to functional redundancy between the CesA6 and CesA6-related CesAs, namely CesA2, 5 and 9.Citation5,14 Similar to the cc1 cc2 pom2-4 triple mutants, the cc1 cc2 prc1-1 triples also showed an aggravating mutant phenotype (), again suggesting that the CC proteins have an important function when members of the CSC are impaired by genetic means. These data add to the functional aspects of the CC proteins and show that they maintain important functions associated with the CSC when cellulose synthesis is impaired.
Taken together, our data indicate that the CC proteins and the CSI1 have different functions at the CSC-microtubule nexus, and that the CC proteins have a role in cellulose synthesis beyond aiding the CSC during salt stress.
Disclosure of Potential Conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The authors are grateful to the Max-Planck Gesellschaft for funding. CK was funded from an IMPRS fellowship via the MPG. Part of the research was funded through the DFG grant PE1642/6-1, the ARC grant DP150103495, and the Hermon-Slade foundation grant # 821372.”
References
- Atmodjo MA, Hao Z, Mohnen D. Evolving views of pectin biosynthesis. Annu Rev Plant Biol 2013; 64:747-79; PMID:23451775; http://dx.doi.org/10.1146/annurev-arplant-042811-105534
- Bringmann M, Li E, Sampathkumar A, Kocabek T, Hauser MT, Persson S. POM-POM2/cellulose synthase interacting1 is essential for the functional association of cellulose synthase and microtubules in Arabidopsis. Plant Cell 2012; 24:163-77; PMID:22294619; http://dx.doi.org/10.1105/tpc.111.093575
- Carpita N, McCann M. The cell wall. In Biochemistry and Molecular Biology of Plants, Buchanan BB, Wilhelm G, Jones RL, eds (Rockville, IL: American Society of Plant Physiologists), 2000:pp. 52-108
- Crowell EF, Bischoff V, Desprez T, Rolland A, Stierhof YD, Schumacher K, Gonneau M, Höfte H, Vernhettes S. Pausing of Golgi bodies on microtubules regulates secretion of cellulose synthase complexes in Arabidopsis. Plant Cell 2009; 21:1141-54; PMID:19376932; http://dx.doi.org/10.1105/tpc.108.065334
- Desprez T, Juraniec M, Crowell EF, Jouy H, Pochylova Z, Parcy F, Höfte H, Gonneau M, Vernhettes S. Organization of cellulose synthase complexes involved in primary cell wall synthesis in Arabidopsis thaliana. Proc Natl Acad Sci U S A 2007; 104:15572-7; PMID:17878303; http://dx.doi.org/10.1073/pnas.0706569104
- Endler A, Kesten C, Schneider R, Zhang Y, Ivakov A, Froehlich A, Funke N, Persson S. A Mechanism for Sustained Cellulose Synthesis during Salt Stress. Cell 2015; 162:1353-64; PMID:26343580; http://dx.doi.org/10.1016/j.cell.2015.08.028
- Gu Y, Kaplinsky N, Bringmann M, Cobb A, Carroll A, Sampathkumar A, Baskin TI, Persson S, Somerville CR. Identification of a cellulose synthase-associated protein required for cellulose biosynthesis. Proc Natl Acad Sci U S A 2010; 107:12866-71; PMID:20616083; http://dx.doi.org/10.1073/pnas.1007092107
- Gutierrez R, Lindeboom JJ, Paredez AR, Emons AM, Ehrhardt DW. Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nat Cell Biol 2009; 11:797-806; PMID:19525940; http://dx.doi.org/10.1038/ncb1886
- Li S, Lei L, Somerville CR, Gu Y. Cellulose synthase interactive protein 1 (CSI1) links microtubules and cellulose synthase complexes. Proc Natl Acad Sci U S A 2012; 109:185-90; PMID:22190487; http://dx.doi.org/10.1073/pnas.1118560109
- Liu L, Shang-Guan K, Zhang B, Liu X, Yan M, Zhang L, Shi Y, Zhang M, Qian Q, Li J, et al. Brittle Culm1, a COBRA-like protein, functions in cellulose assembly through binding cellulose microfibrils. PLoS Genet 2013; 9:e1003704; PMID:23990797; http://dx.doi.org/10.1371/journal.pgen.1003704
- Luo Y, Scholl S, Doering A, Zhang Y, Irani NG, Di Rubbo S, Neumetzler L, Krishnamoorthy P, Van Houtte I, Mylle E, et al. V-ATPase activity in the TGN/EE is required for exocytosis and recycling in Arabidopsis. Nat Plants 2015; 1:15094; http://dx.doi.org/10.1038/nplants.2015.94
- McFarlane HE, Döring A, Persson S. The cell biology of cellulose synthesis. Annu Rev Plant Biol 2014; 65:69-94; PMID:24579997; http://dx.doi.org/10.1146/annurev-arplant-050213-040240
- Nicol F, His I, Jauneau A, Vernhettes S, Canut H, Höfte H. A plasma membrane-bound putative endo-1,4-β-D-glucanase is required for normal wall assembly and cell elongation in Arabidopsis. EMBO J 1998; 17:5563-76; PMID:9755157; http://dx.doi.org/10.1093/emboj/17.19.5563
- Persson S, Paredez A, Carroll A, Palsdottir H, Doblin M, Poindexter P, Khitrov N, Auer M, Somerville CR. Genetic evidence for three unique components in primary cell-wall cellulose synthase complexes in Arabidopsis. Proc Natl Acad Sci U S A 2007; 104:15566-71; PMID:17878302; http://dx.doi.org/10.1073/pnas.0706592104
- Roudier F, Fernandez AG, Fujita M, Himmelspach R, Borner GH, Schindelman G, Song S, Baskin TI, Dupree P, Wasteneys GO, et al. COBRA, an Arabidopsis extracellular glycosyl-phosphatidyl inositol-anchored protein, specifically controls highly anisotropic expansion through its involvement in cellulose microfibril orientation. Plant Cell 2005; 17:1749-63; PMID:15849274; http://dx.doi.org/10.1105/tpc.105.031732
- Sánchez-Rodríguez C, Bauer S, Hématy K, Saxe F, Ibáñez AB, Vodermaier V, Konlechner C, Sampathkumar A, Rüggeberg M, Aichinger E, et al. Chitinase-like1/pom-pom1 and its homolog CTL2 are glucan-interacting proteins important for cellulose biosynthesis in Arabidopsis. Plant Cell 2012; 24:589-607; PMID: 22327741; http://dx.doi.org/10.1105/tpc.111.094672
- Scheller HV, Ulvskov P. Hemicelluloses. Annu Rev Plant Biol 2010; 61:263-89; PMID:20192742; http://dx.doi.org/10.1146/annurev-arplant-042809-112315
- Somerville C, Bauer S, Brininstool G, Facette M, Hamann T, Milne J, Osborne E, Paredez A, Persson S, Raab T, et al. Toward a systems approach to understanding plant cell walls. Science 2004; 306:2206-11; PMID:15618507; http://dx.doi.org/10.1126/science.1102765
- Vain T, Crowell EF, Timpano H, Biot E, Desprez T, Mansoori N, Trindade LM, Pagant S, Robert S, Höfte H, et al. The Cellulase KORRIGAN Is Part of the Cellulose Synthase Complex. Plant Physiol 2014; 165:1521-32; PMID:24948829; http://dx.doi.org/10.1104/pp.114.241216
