ABSTRACT
Natural pyrethrins are used to control household and agricultural pests, and it is of value to understand biosynthesis in Tanacetum cinerariifolium for enhanced production. We previously found that a blend of four green leaf volatiles (GLVs) and (E)-β-farnesene emitted by T. cinerariifolium seedlings enhanced gene expressions of certain biosynthetic enzymes in unwounded seedlings; however, the extent to which such a regulation facilitates pyrethrin biosynthesis remains unknown. Here we have investigated the effects of the blend of the volatile organic compounds (VOCs) on gene expressions of seven biosynthetic enzymes. VOC treatment resulted in enhanced chrysanthemyl diphosphate synthase (CDS), chrysanthemic acid synthase (CAS), Tanacetum cinerariifolium GDSL lipase (TcGLIP) and acyl-Coenzyme A oxidase 1 (ACX1) gene expressions that reached a peak at a 12 h VOC treatment, whereas the treatment minimally influenced the expressions of other biosynthetic genes. In undifferentiated Tanacetum tissues, such VOC-induced amplification of CDS, CAS, TcGLIP and ACX1 gene expressions were markedly reduced, suggesting that a high-resolution, VOC-mediated communication is an event selective to differentiated plants.
Abbreviations
| ACX1 | = | acyl-Coenzyme A oxidase 1 |
| AOC | = | allene oxide cyclase |
| AOS | = | allene oxide synthase |
| CAS | = | chrysanthemic acid synthase |
| CDS | = | chrysanthemyl diphosphate synthase |
| CoA ligase | = | chrysanthemic acid:coenzyme A ligase |
| HPL, | = | hydroperoxide lyase |
| JA | = | 7-iso-jasmonic acid |
| 13-LOX | = | 13-lipoxygenase |
| OPC-8 | = | 3-oxo-2-(2-pentenyl)-cyclopentane-1-octanoic acid |
| OPDA | = | 12-oxo-10,15-phytodienoic acid |
| OPR3 | = | OPDA reductase 3 |
| TcGLIP | = | Tanacetum cinerariifolium GDSL lipase |
Introduction
Plants emit specific blends of volatile organic compounds (VOCs) in response to infestation by herbivores. When receiver plants eavesdrop the conditions of their neighboring infested plants in terms of herbivore-induced plant VOCs (HIPVs), they induce defense gene expressions to increase resistance to herbivory.Citation1-6 HIPVs include terpenes,Citation4,5 green leaf volatiles (GLVs),Citation7 indole,Citation8 methyl jasmonateCitation9 and methyl salicylate.Citation10,11
In the case of Tanacetum cinerariifolium, which produces insecticides pyrethrins by 2 different pathways ()Citation12 as the current mainly defense mechanism,Citation13 uninfested seedlings exposed to volatiles from artificially damaged conspecies increased the amounts of pyrethrins.Citation14 Mechanically wounded leaves mainly emit GLVsCitation7 and (E)-β-farnesene as induced VOCs. Notably, emission of (Z)-3-hexenal, (E)-2-hexenal and (Z)-3-hexenol was found to increase rapidly upon wounding, whereas that of (Z)-3-hexenyl acetate and (E)-β-farnesene increased more slowly, reaching a peak approximately 35–65 min after wounding.Citation14 We prepared a blend of these 5 VOCs according to the gas chromatography coupled to mass spectrometry (GC-MS) profile to examine their effects on several biosynthetic gene expressions in unwounded seedlings of T. cinerariifolium. For the enzymes involved in the acid moiety biosynthesis, gene expression of 1-deoxy-d-xylulose 5-phosphate synthase (DXS) significantly increased at 3 h after the start of VOC treatment and decreased thereafter, whereas gene expression of chrysanthemyl diphosphate synthase (CDS) was amplified more slowly. In contrast, much weaker or no significant VOC sensitivity was observed on the gene expression of lipoxygenase (LOX) and allene oxide synthase (AOS) involved in the alcohol moiety biosynthesis. Furthermore, it was found that the 5 VOC components were essential for the enhanced gene expression of DXS and CDS.
Figure 1. Biosynthetic pathways to pyrethrin I in Tanacetum cinerariifolium. Compound names are indicated in the structures, while enzyme names are shown in BOLD alongside each arrow. Abbreviations (compound names): OPDA, 12-oxo-phytodienoic acid; OPC-8, 3-oxo-2-(2-pentenyl)-cyclopentane-1-octanoic acid. Abbreviations (enzyme names): ACX1, acyl-Coenzyme A oxidase 1; AOC, allene oxide cyclase; AOS, allene oxide synthase; CAS, chrysanthemic acid synthase; CDS, chrysanthemic diphosphate synthase; CoA ligase, chrysanthemic acid:coenzyme A ligase; HPL, hydroperoxide lyase; 13-LOX, 13-lipoxygenase; OPR3, 3-oxo-2-(2-pentenyl)-cyclopentane-1-octanoic acid reductase 3; TcGLIP, Tanacetum cinerariifolium GDSL lipase. To date, no gene underlying a conversion of 7-iso-jasmonic acid to cis-jasmone has been identified, whereas iso-OPDA has been shown to possibly underlie the cis-jasmone biosynthesis.Citation23.
These earlier results raise a question regarding how selectively the specific blend of the five VOCs enhances expressions of the other biosynthetic genes or candidates. Here we investigated the effects of the four GLVs and (E)-β-farnesene mixture on gene expressions of seven biosynthetic enzymes at 6, 12 and 24 h after the initiation of VOC treatment. In addition, we investigated the gene expressions in undifferentiated Tanacetum tissues exposed to VOCs to show the necessity of differentiated tissues for the highly sensitive perception of the airborne signals.
Materials and methods
Plant samples
All plants and tissues were grown at 25°C under a 16:8 h day: night cycle with an illumination of 5,000 lux. Callus tissues were induced from leaves of young seedlings by growing on Murashige–Skoog agar plates supplemented with 2,4-dichlorophenoxy acetic acid (1 mg/mL) and benzyladenine (1 mg/mL).
VOC treatment
Young seedlings and callus tissues of T. cinerariifolium were treated for 6, 12 and 24 h under a constant illumination of 5,000 lux with a blend of 90.0 nM (Z)-3-hexenal, 0.5 nM (E)-2-hexenal, 5.0 nM (Z)-3-hexen-1-ol, 6.0 nM (Z)-3-hexenyl acetate and 13.0 nM (E)-β-farnesene which was vaporised from a cotton swab in a tightly sealed 3 L glass vessel, whereas vehicle methanol was treated as control, as described previously.Citation14
Real time polymerase chain reaction
Total RNA of the plant samples were extracted using a TRIZol reagent (Thermo Fisher Scientific, Waltham, MA, USA) and genomic DNA was removed using a DNA-free Kit (Thermo Fisher Scientific). The RNA samples were reverse-transcribed using a PrimeScript RT Reagent Kit (Perfect Real Time; Takara Bio, Shiga, Japan) and real time polymerase chain reaction (real time-PCR) was conducted using a FastStart Universal SYBR Green Master (Rox; F. Hoffmann-La Roche, Basel, Switzerland). For developing a T. cinerariifolium EST database, we carried out RNA-Seq analyses using next-generation sequencing instruments Hiseq1000 and Hiseq1500 (Illumina, San Diego, CA, USA) at the Kazusa DNA Research Institute. The assembled sequence data, as well as the GenBank sequence data of chrysanthemic acid synthase (CAS, accession number: KC441525.1) and T. cinerariifolium GDSL lipase (TcGLIP, accession number: JN418994), were used to design primers for real time PCR as follows: CDS (Forward 5′-TGTACCTGGAGGAAAGATGGTCC-3′, reverse 5′-CAACCAAGAGCACACGCGAG-3′; CAS (Forward, 5′-TTGGGTCAGGAAGGAGAATG-3′; reverse, 5′-CATCAGGGAGTTTCCAATCG-3′), TcGLIP (Forward, 5′-ATGGCTGTTGCAAGCAGAAAACTGGG-3′; 5′-CAGCAGCTTGTTGAGAACTAAGGC-3′), AOS (Forward, 5′-AAGACGGAGTAACGGCCCTAAG-3′; reverse, 5′-GACGTTGGTGAACAAACGGC-3′), allene oxide cyclase (AOC; Forward, 5′-GGTACTGGGATATTTGCTGGAGCA-3′; reverse, 5′-GAGTCACCGGGGTAACTAACAACT-3′), OPDA reductase 3 (OPR3; Forward, 5′-GCAGACCGAGTTGGTCTTAGAATC-3′, reverse, 5′-TTGACCATAAGCGGTGTAACGTG-3′) and acyl-Coenzyme A oxidase 1 (ACX1; Forward, 5′-AACTACCGCAACAGCTGATGG-3′, reverse, 5′-CCAGCTCAGAGACCGTCTTAAC-3′). For the actin gene, the forward and reverse primers were designed as 5′-CCTTCCACATGCCATGCCATCCTTCG-3′ and 5′-CCGTTCTGCTGTAGTGGTGAAC-3′, respectively. PCR was performed at 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. Each gene expression was indicated as a relative value to the control actin gene expression.
Results
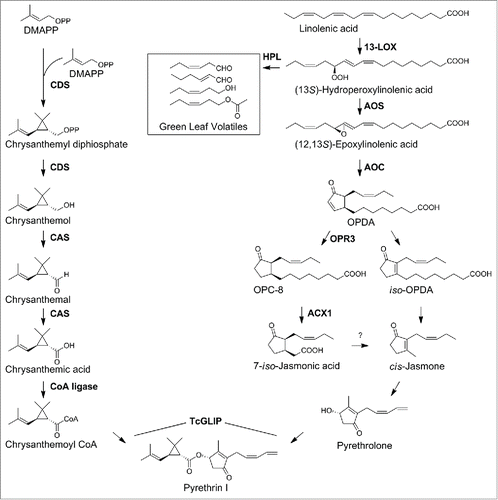
The acid and alcohol moieties of pyrethrins were biosynthesised by two different pathwaysCitation12,15 and finally converged by a GDSL lipase, TcGLIP.Citation16 Of the enzymes shown in , we investigated gene expressions of CDS, CAS, TcGLIP, AOS, AOC, OPR3 and ACX1 in response to gaseous treatments to the seedlings with the specific blend of GLVs and (E)-β-farnesene for 6, 12 and 24 h (). When treated with the VOC blend for 6 h, CDS, CAS and TcGLIP showed a tendency of gene amplification by VOC treatment; however, only the change in CAS gene expression was significant (). Treating intact Tanacetum seedlings for 12 h resulted in a broader range of biosynthetic gene expressions (). The CDS and CAS genes were amplified by 147- and 158-fold, respectively, followed by TcGLIP which was amplified by 29-fold, whereas the ACX1 gene was amplified 3-fold. The AOS gene appeared to be amplified but its change was not statistically significant. Further the prolongation of VOC treatment to 24 h resulted in reduced expressions of all genes investigated to the control level ().
Figure 2. Gene expressions relevant to pyrethrin biosynthesis in response to wound-induced volatile organic compounds blend treatment for 6 (A), 12 (B) and 24 h (C) in intact Tanacetum cinerariifolium leaves. Each bar graph represents mean ± standard error of the mean (n = 3). The difference was analyzed by t-test after conversion of the relative gene expression data using the logarithm function (*, p < 0.05).
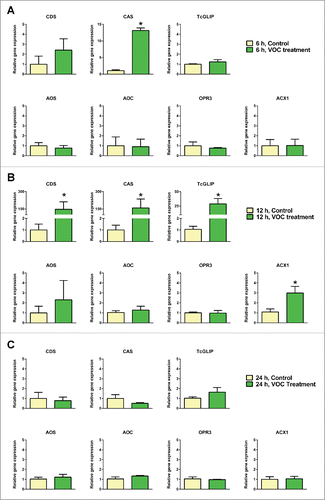
To examine whether VOC-induced gene expression is limited to differentiated tissue, we induced callus tissues from Tanacetum leaf fragments by treating with the plant hormones 2,4-dichlorophenoxy acetic acid and benzyladenine (). We treated the undifferentiated tissues with the VOC blend for 12 h, and measured the highest VOC-induced gene expressions in leaves. Gene expressions of CDS, CAS, TcGLIP and ACX1 were enhanced, with a lower amplification ratio than that in the intact leaves ().
Figure 3. Effects of the wound-induce volatile organic compounds blend on callus tissues derived from Tanacetum cinerariifolium leaves. Each bar graph represents mean ± standard error of the mean (n = 4). The difference was analyzed by t-test after conversion of the relative gene expression data using the logarithm function (*, p < 0.05).
Discussion
The current study was the first to investigate the effects of the blend of four kinds of GLVs and (E)-β-farnesene on seven genes relevant to pyrethrin biosynthesis in T. cinerariifolium. The wound induced VOCs did not universally enhance expressions of all the biosynthetic genes to an equivalent level; however, selective enhancement of four genes occurred (). In addition, VOC-induced amplification of the target gene was not equal. The CDS and CAS genes, which are involved in the acid moiety biosynthesis, showed the highest VOC sensitivity of the genes investigated (). CDS is localized in the trichomeCitation17,18; thus, CAS and the VOC perception machinery may be co-localized in the same organ. Even these two gene expressions showed a transient VOC-induced amplification, reaching a peak at 12 h with no significant change compared with the control at 24 h (). The reduced gene expressions of CDS and CAS at 24 h as compared with those at 12 h are reasonable because the plants were at no risk of herbivore attack during the experiments; thus, there was no necessity for continuous pyrethrin biosynthesis. Furthermore, the TcGLIP gene involved in the ester bond formation in pyrethrin biosynthesis was markedly increased in response to VOC treatment (), suggesting its important role in the insect defense and a strong correlation with the CDS and CAS in pyrethrin biosynthesis.
The genes relevant to the alcohol moiety biosynthesis showed low or no significant amplification in the case of ACX1 and the other genes, respectively (), when exposed to the wound induced VOCs. If the airborne signals preferentially control pyrethrin biosynthesis, an interpretation of the low VOC sensitivity of AOS, AOC, OPR3 and ACX1 is that their roles in the biosynthesis of a plant hormone jasmonic acid and its amino acid conjugatesCitation19-22 are higher than those in pyrethrin biosynthesis, thereby showing a lower VOC-sensitivity than CDS, CAS and TcGLIP that are directly involved in pyrethrin biosynthesis. In addition, because of the evidence for conversion of iso-OPDA to a possible pyrethrolone precursor cis-jasmone in other plantsCitation23 (), it is reasonable to consider that the OPR3 gene contribution to pyrethrin biosynthesis is less; thus, its VOC sensitivity is less.()
To date, intensive studies have investigated the effects of plant derived VOCs on differentiated plants to establish their diverse roles in plant–plant communications.Citation1-6 However, to the best of our knowledge, no airborne signals have been tested as gas on undifferentiated tissues in terms of gene expression. Furthermore, CDS, CAS, TcGLIP and ACX1 retain their VOC sensitivity, even in the undifferentiated tissue (). However, the degree of VOC-induced gene amplification was lower than that observed in the undifferentiated tissue (), probably because of down-regulated signal transduction from VOC perception to gene transcription in the callus.
In conclusion, we found that the wound-induced VOCs selectively regulated certain enzyme genes underlying pyrethrin biosynthesis in the intact seedling leaves of T. cinerariifolium. Of the genes investigated, CDS, CAS and TcGLIP gene expression was markedly enhanced by VOC treatment, with higher amplification than in the undifferentiated tissue, indicating a requirement of differentiated leaves for the highly-sensitive plant-plant communications.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by Grants-in-Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Science [grant number 25282234 (KM), 26350967 (HS)] and the Strategic Project to Support the Formation of Research Bases at Private Universities: Matching Fund Subsidy from Ministry of Education, Culture, Sports, Science and Technology [grant number S1101035].
References
- Loreto F, Dicke M, Schnitzler JP, Turlings TC. Plant volatiles and the environment. Plant Cell Environ 2014; 37:1905-8; PMID:24811745; http://dx.doi.org/10.1111/pce.12369
- Heil M, Karban R. Explaining evolution of plant communication by airborne signals. Trends Ecol Evol 2010; 25:137-44; PMID:19837476; http://dx.doi.org/10.1016/j.tree.2009.09.010
- Dicke M, Baldwin IT. The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci 2010; 15:167-75; PMID:20047849; http://dx.doi.org/10.1016/j.tplants.2009.12.002
- Arimura G, Matsui K, Takabayashi J. Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol 2009; 50:911-23; PMID:19246460; http://dx.doi.org/10.1093/pcp/pcp030
- Okada K, Abe H, Arimura G. Jasmonates induce both defence responses and communication in monocotyledonous and dicotyledonous plants. Plant Cell Physiol 2015; 56:16-27; PMID:25378688; http://dx.doi.org/10.1093/pcp/pcu158
- Ueda H, Kikuta Y, Matsuda K. Plant communication: mediated by individual or blended VOCs? Plant Signal Behav 2012; 7:222-6; PMID:22353877; http://dx.doi.org/10.4161/psb.18765
- Matsui K. Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr Opin Plant Biol 2006; 9:274-80; PMID:16595187; http://dx.doi.org/10.1016/j.pbi.2006.03.002
- Erb M, Veyrat N, Robert CA, Xu H, Frey M, Ton J, Turlings TC. Indole is an essential herbivore-induced volatile priming signal in maize. Nat Commun 2015; 6:6273; PMID:25683900; http://dx.doi.org/10.1038/ncomms7273
- Farmer EE, Ryan CA. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci U S A 1990; 87:7713-6; PMID:11607107; http://dx.doi.org/10.1073/pnas.87.19.7713
- Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 2000; 406:512-5; PMID:10952311; http://dx.doi.org/10.1038/35020072
- Ozawa R, Arimura G, Takabayashi J, Shimoda T, Nishioka T. Involvement of jasmonate- and salicylate-related signaling pathways for the production of specific herbivore-induced volatiles in plants. Plant Cell Physiol 2000; 41:391-8; PMID:10845451; http://dx.doi.org/10.1093/pcp/41.4.391
- Matsuda K. Pyrethrin biosynthesis and its regulation in Chrysanthemum cinerariaefolium. Top Curr Chem 2012; 314:73-81; PMID:22006239; http://dx.doi.org/10.1007/128_2011_271
- Yang T, Stoopen G, Wiegers G, Mao J, Wang C, Dicke M, Jongsma MA. Pyrethrins protect pyrethrum leaves against attack by western flower thrips, Frankliniella occidentalis. J Chem Ecol 2012; 38:370-7; PMID:22456949; http://dx.doi.org/10.1007/s 10886-012-0097-7
- Kikuta Y, Ueda H, Nakayama K, Katsuda Y, Ozawa R, Takabayashi J, Hatanaka A, Matsuda K. Specific regulation of pyrethrin biosynthesis in Chrysanthemum cinerariaefolium by a blend of volatiles emitted from artificially damaged conspecific plants. Plant Cell Physiol 2011; 52:588-96; PMID:21296762; http://dx.doi.org/10.1093/pcp/pcr017
- Matsuda K, Kikuta Y, Haba A, Nakayama K, Katsuda Y, Hatanaka A, Komai K. Biosynthesis of pyrethrin I in seedlings of Chrysanthemum cinerariaefolium. Phytochemistry 2005; 66:1529-35; PMID:15964038; http://dx.doi.org/10.1016/j.phytochem.2005.05.005
- Kikuta Y, Ueda H, Takahashi M, Mitsumori T, Yamada G, Sakamori K, Takeda K, Furutani S, Nakayama K, Katsuda Y, et al. Identification and characterization of a GDSL lipase-like protein that catalyzes the ester-forming reaction for pyrethrin biosynthesis in Tanacetum cinerariifolium- a new target for plant protection. Plant J 2012; 71:183-93; PMID:22385412; http://dx.doi.org/10.1111/j.1365-313X.2012.04980.x
- Ramirez AM, Stoopen G, Menzel TR, Gols R, Bouwmeester HJ, Dicke M, Jongsma MA. Bidirectional secretions from glandular trichomes of pyrethrum enable immunization of seedlings. Plant Cell 2012; 24:4252-65; PMID:23104830; http://dx.doi.org/10.1105/tpc.112.105031
- Yang T, Gao L, Hu H, Stoopen G, Wang C, Jongsma MA. Chrysanthemyl diphosphate synthase operates in planta as a bifunctional enzyme with chrysanthemol synthase activity. J Biol Chem 2014; 289:36325-35; PMID:25378387; http://dx.doi.org/10.1074/jbc.M114.623348
- Fonseca S, Chico JM, Solano R. The jasmonate pathway: the ligand, the receptor and the core signalling module. Curr Opin Plant Biol 2009; 12:539-47; PMID:19716757; http://dx.doi.org/10.1016/j.pbi.2009.07.013
- Staswick PE, Tiryaki I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 2004; 16:2117-27; PMID:15258265; http://dx.doi.org/10.1105/tpc.104.023549
- Wasternack C, Hause B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot 2013; 111:1021-58; PMID:23558912; http://dx.doi.org/10.1093/aob/mct067
- Schaller A, Stintzi A. Enzymes in jasmonate biosynthesis - structure, function, regulation. Phytochemistry 2009; 70:1532-8; PMID:19703696; http://dx.doi.org/10.1016/j.phytochem.2009.07.032
- Dabrowska P, Boland W. iso-OPDA: an early precursor of cis-jasmone in plants? Chembiochem 2007; 8:2281-5; PMID:18033720; http://dx.doi.org/10.1002/cbic.200700464
