ABSTRACT
Myrosin cells accumulate myrosinases in their vacuoles to catalyze the production of toxic compounds when tissues are damaged by herbivores. Myrosin cells are positioned adjacent to the abaxial side of the vasculature but their origin is unclear. To determine whether the myrosin cells are differentiated from vascular precursor cells, we generated a transgenic Arabidopsis line that expressed a myrosin cell reporter together with one of 3 vascular precursor cell reporters. The myrosin-positive cells were discontinuously distributed while the vascular precursor-positive cells were continuously distributed. The fluorescent signals of the myosin and vascular reporters did not overlap. Furthermore, the shape of myrosin-positive cells was different from the shape of vascular precursor-positive cells. These results indicate that myosin cells develop independently of the vasculature.
Abbreviations:
| ARF5 | = | AUXIN RESPONSE FACTOR 5 |
| AtHB8 | = | ARABIDOPSIS THALIANA HOMEOBOX GENE 8 |
| DAG | = | days after germination |
| MP | = | MONOPTEROS |
| PIN | = | PIN-FORMED |
| RFP | = | red fluorescent protein |
| TGG | = | thioglucoside glucohydrolase |
Introduction
The myrosinase–glucosinolate system is a chemical defense system against herbivory in Brassicales. Myrosinases and their substrates, glucosinolates, are stored separately in myrosin cells and S-cells, respectively.Citation1-6 When herbivores damage plant tissues, myrosinase released from myrosin cells catalyzes hydrolysis of glucosinolates to produce toxic compounds against herbivores.Citation6-11 A characteristic feature of myrosin cells is their distribution along the leaf vasculature.Citation2-4,12-14 Myrosin cells specifically develop adjacent to phloem cells (on the abaxial side of vasculature) and are called myrosin phloem cells.Citation3,6 Therefore, myrosin cells are assumed to be a type of vascular cells, although the direct evidence is lacking.
Recently, the basic helix–loop–helix transcription factor FAMA was identified as a master regulator of myrosin cell differentiation.Citation15,16 In the inner tissues of leaf primordia, cells that express FAMA are morphologically indistinguishable from neighboring ground meristem cells,Citation15,16 which are known to be mother cells of all inner leaf cells, including vascular precursor cells.Citation17-19 The expression pattern of FAMA differs from those of vascular precursor genes.Citation15,16 To determine whether myrosin cells are differentiated independent of vascular cells, in this study, we simultaneously examined expression patterns of a myrosin cell reporter and vascular precursor reporters. The present results demonstrate that myrosin cells are differentiated directly from ground meristem cells rather than vascular precursor cells.
Results
We generated transgenic plants that co-expressed the myrosin cell reporter ProTGG2:VENUS-2sc, which labels endoplasmic reticulum (ER) and vacuoles,Citation20 and a vascular precursor cell reporter, Q0990, which labels ER,Citation21 and examined changes in expression patterns of these reporters during development of juvenile leaves. Three days after germination (DAG), 2 cells that expressed the myrosin cell reporter emerged independently near the middle of the future primary vein in leaf primordia (),Citation22 whereas several cells that expressed the Q0990 reporter of vascular precursor cells aligned along the future primary vein ().Citation21 Importantly, the VENUS-2sc signals of myrosin cells and the Q0990 signals of vascular precursor cells were near each other but did not overlap (). At DAG4.5 and DAG7, more cells that expressed the myrosin cell reporter discontinuously aligned near the future vein network (). The distribution pattern of the myrosin cell reporter was distinctly different from the continuous distribution pattern of the vascular precursor cell reporter Q0990 (), and their fluorescent signals did not overlap (), which indicates that the myrosin cells are not differentiated from the Q0990-positive vascular precursor cells.
Figure 1. Spatiotemporal patterns during leaf development differ between the myrosin cell reporter and the vascular precursor cell reporter Q0990. The abaxial side of a juvenile leaf in a transgenic plant that expresses both the myrosin cell reporter ProTGG2:VENUS-2sc and the vascular precursor cell reporter Q0990. Fluorescent images of myrosin cells (green; [A], [E], and [I]) and vascular precursor cells (magenta; [B], [F], and [J]) at DAG3 (upper panels), DAG4.5 (middle panels), and DAG7 (lower panels). Merged images of the leaves are shown ([C], [G], and [K]). Schematic representations show vascular precursor development (magenta) in the juvenile leaf at DAG3 (D), DAG4.5 (H), and DAG7 (L). Myrosin cells are indicated by arrows in (A). The green fluorescent punctate structures are guard cells (indicated by arrowheads in [E]). Bars = 50 μm in (A) to (H), and 200 μm in (I) to (L).
![Figure 1. Spatiotemporal patterns during leaf development differ between the myrosin cell reporter and the vascular precursor cell reporter Q0990. The abaxial side of a juvenile leaf in a transgenic plant that expresses both the myrosin cell reporter ProTGG2:VENUS-2sc and the vascular precursor cell reporter Q0990. Fluorescent images of myrosin cells (green; [A], [E], and [I]) and vascular precursor cells (magenta; [B], [F], and [J]) at DAG3 (upper panels), DAG4.5 (middle panels), and DAG7 (lower panels). Merged images of the leaves are shown ([C], [G], and [K]). Schematic representations show vascular precursor development (magenta) in the juvenile leaf at DAG3 (D), DAG4.5 (H), and DAG7 (L). Myrosin cells are indicated by arrows in (A). The green fluorescent punctate structures are guard cells (indicated by arrowheads in [E]). Bars = 50 μm in (A) to (H), and 200 μm in (I) to (L).](/cms/asset/5d9061b7-0676-42ae-a507-587fc0e77c89/kpsb_a_1150403_f0001_oc.gif)
To exclude the possibility that the myrosin cells are differentiated from Q0990-negative vascular precursor cells, we generated transgenic plants that co-expressed the myrosin cell reporter ProTGG2:VENUS-2sc and one of the vascular precursor cell reporters, ProAtHB8:TagRFP, which labels cytosol, or J1721, which labels ER.Citation21 ProAtHB8:TagRFP and J1721 are concurrently expressed in vascular precursor cells, and their expression is slightly earlier than that of Q0990 during vascular cell development.Citation21
We compared the expression pattern of ProTGG2:VENUS-2sc with those of ProAtHB8:TagRFP and J1721 around future secondary veins at DAG4-DAG5. Myrosin cells that expressed ProTGG2:VENUS-2sc were distributed near the vascular precursor cells that expressed ProAtHB8:TagRFP () or J1721 (). However, we did not find cells that expressed both the myrosin cell reporter and either vascular precursor cell reporter (ProAtHB8:TagRFP, ; J1721, ), as was observed with Q0990 (). In addition, cell shapes were substantially different between the myrosin cells that expressed ProTGG2:VENUS-2sc and the vascular precursor cells that expressed either ProAtHB8:TagRFP or J1721 (). We did not detect any cells that expressed both the myrosin cell reporter and a vascular precursor reporter at any stage; this indicates that myrosin cells are not differentiated from vascular precursor cells.
Figure 2. Myrosin cells and vascular precursor cells differ in spatial pattern and cell shape at an early stage of differentiation. Fluorescent images of myrosin cells (green; [A], [D], and [G]) and vascular precursor cells (magenta; [B], [E], and [H]), and their merged images ([C], [F], and [I]) at future secondary veins in a juvenile leaf at DAG4 or DAG5 in plants that express a myrosin cell marker (ProTGG2:VENUS-2sc) and each of the 3 vascular precursor cell markers: Q0990 (B), ProAtHB8:TagRFP (E), and J1721 (H). Arrowheads in (A) indicate stomata. Bars = 50 μm in (A) to (C), and 20 μm in (D) to (I).
![Figure 2. Myrosin cells and vascular precursor cells differ in spatial pattern and cell shape at an early stage of differentiation. Fluorescent images of myrosin cells (green; [A], [D], and [G]) and vascular precursor cells (magenta; [B], [E], and [H]), and their merged images ([C], [F], and [I]) at future secondary veins in a juvenile leaf at DAG4 or DAG5 in plants that express a myrosin cell marker (ProTGG2:VENUS-2sc) and each of the 3 vascular precursor cell markers: Q0990 (B), ProAtHB8:TagRFP (E), and J1721 (H). Arrowheads in (A) indicate stomata. Bars = 50 μm in (A) to (C), and 20 μm in (D) to (I).](/cms/asset/e2e14285-73fc-40f1-8d90-972aaa3d51dc/kpsb_a_1150403_f0002_oc.gif)
Discussion
In this study, we showed that myrosin cells are not differentiated from vascular precursor cells. Considering that the master regulator of myrosin cell development, FAMA, is expressed in cells morphologically indistinguishable from neighboring ground meristem cells,Citation15,16 we conclude that myrosin cells are directly differentiated from ground meristem cells (). Our findings support the idea that myrosin cells are not a type of the phloem, which is surprising because myrosin cells directly face phloem and, in some cases, appear to be partially surrounded by phloem parenchyma cells.
Figure 3. Model of myrosin cell differentiation. Myrosin cells are differentiated directly from ground meristem cells by expressing the bHLH transcription factor FAMA, whereas vascular cells are differentiate from another subset of ground meristem cells by expressing AtHB8.
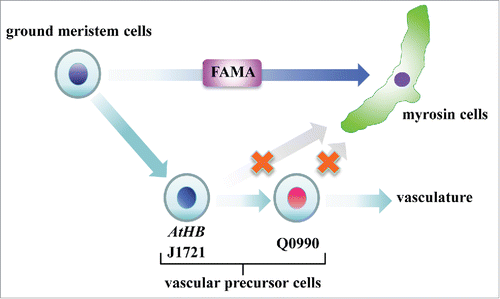
How plants can form such a distribution pattern is not yet fully understood. A transcription factor, MONOPTEROS (MP)/AUXIN RESPONSE FACTOR 5 (ARF5), is widely expressed in ground meristem cells. During differentiation of vascular precursor cells, AtHB8 starts to be expressed in a subset of MP/ARF5-positive ground meristem cells.Citation23 One possibility is that myrosin cells are differentiated from MP/ARF5-positive and AtHB8-negative ground meristem cells. We previously identified the Arabidopsis mutant syntaxin of plants 22 (syp22), which exhibits an increased number of myrosin cells and a simpler vascular pattern.Citation4,22,24,25 This phenotype is caused by non-polar localization of the auxin efflux carrier PIN-FORMED 1 (PIN1) and abnormal distribution of auxin in the leaf primodia.Citation22,24 It is possible that PIN1 polarity and auxin flux regulate the balance of cell differentiation from ground meristem cells into myrosin cells or vascular cells.
In stomatal lineage cells, stepwise expression of the homologous bHLH genes SPEECHLESS (SPCH), MUTE, and FAMA regulate stomatal development.Citation26-30 Unlike FAMA, SPCH and MUTE are not required for myrosin cell development.Citation15,16 However, the entry of ground meristem cells into the myrosin cell lineage may be regulated by yet unknown factor(s) that are expressed prior to FAMA. Consequently, a forward genetic approach would be useful to isolate the unknown factor(s) and may provide insight into the mechanism underlying myrosin cell differentiation.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the Nottingham Arabidopsis Stock Center for providing seeds of A. thaliana lines.
Funding
This work was supported by Specially Promoted Research of a Grant-in-Aid for Scientific Research to I.H-.N. (no. 22000014), Grants-in-Aid for Scientific Research to I.H-.N. (no. 15H05776) and to H.U. (nos. 25440132 and 15KT0151), a Research Fellowship for Young Scientists to M.S. (no. 24005453), and a Postdoctoral Fellowship for Research Abroad to M.S. from the Japan Society for the Promotion of Science (JSPS).
References
- Koroleva OA, Davies A, Deeken R, Thorpe MR, Tomos AD, Hedrich R. Identification of a new glucosinolate-rich cell type in Arabidopsis flower stalk. Plant Physiol 2000; 124:599-608; PMID:11027710; http://dx.doi.org/10.1104/pp.124.2.599
- Andreasson E, Bolt Jorgensen L, Hoglund AS, Rask L, Meijer J. Different myrosinase and idioblast distribution in Arabidopsis and Brassica napus. Plant Physiol 2001; 127:1750-1763; PMID:11743118; http://dx.doi.org/10.1104/pp.010334
- Husebye H, Chadchawan S, Winge P, Thangstad OP, Bones AM. Guard cell- and phloem idioblast-specific expression of thioglucoside glucohydrolase 1 (myrosinase) in Arabidopsis. Plant Physiol 2002; 128:1180-1188; PMID:11950967; http://dx.doi.org/10.1104/pp.010925
- Ueda H, Nishiyama C, Shimada T, Koumoto Y, Hayashi Y, Kondo M, Takahashi T, Ohtomo I, Nishimura M, Hara-Nishimura I. AtVAM3 is required for normal specification of idioblasts, myrosin cells. Plant Cell Physiol 2006; 47:164-175; PMID:16306062; http://dx.doi.org/10.1093/pcp/pci232
- Shroff R, Vergara F, Muck A, Svatos A, Gershenzon J. Nonuniform distribution of glucosinolates in Arabidopsis thaliana leaves has important consequences for plant defense. Proc Natl Acad Sci USA 2008; 105:6196-6201; PMID:18408160; http://dx.doi.org/10.1073/pnas.0711730105
- Kissen R, Rossiter JT, Bones AM. The ‘mustard oil bomb’: not so easy to assemble?! Localization, expression and distribution of the components of the myrosinase enzyme system. Phytochem Rev 2009; 8:69-86; http://dx.doi.org/10.1007/s11101-008-9109-1
- Rask L, Andreasson E, Ekbom B, Eriksson S, Pontoppidan B, Meijer J. Myrosinase: gene family evolution and herbivore defense in Brassicaceae. Plant Mol Biol 2000; 42:93-113; PMID:10688132; http://dx.doi.org/10.1023/A:1006380021658
- Wittstock U, Halkier BA. Glucosinolate research in the Arabidopsis era. Trends Plant Sci 2002; 7:263-270; PMID:12049923; http://dx.doi.org/10.1016/S1360-1385(02)02273-2
- Grubb CD, Abel S. Glucosinolate metabolism and its control. Trends Plant Sci 2006; 11:89-100; PMID:16406306; http://dx.doi.org/10.1016/j.tplants.2005.12.006
- Halkier BA, Gershenzon J. Biology and biochemistry of glucosinolates. Annu Rev Plant Biol 2006; 57:303-333; PMID:16669764; http://dx.doi.org/10.1146/annurev.arplant.57.032905.105228
- Hopkins RJ, van Dam NM, van Loon JJ. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu Rev Entomol 2009; 54:57-83; PMID:18811249; http://dx.doi.org/10.1146/annurev.ento.54.110807.090623
- Xue J, Jorgensen M, Pihlgren U, Rask L. The myrosinase gene family in Arabidopsis thaliana: gene organization, expression and evolution. Plant Mol Biol 1995; 27:911-922; PMID:7766881; http://dx.doi.org/10.1007/BF00037019
- Thangstad OP, Gilde B, Chadchawan S, Seem M, Husebye H, Bradley D, Bones AM. Cell specific, cross-species expression of myrosinases in Brassica napus, Arabidopsis thaliana and Nicotiana tabacum. Plant Mol. Biol 2004; PMID:15316292; 54:597-611; http://dx.doi.org/10.1023/B:PLAN.0000038272.99590.10
- Barth C, Jander G. Arabidopsis myrosinases TGG1 and TGG2 have redundant function in glucosinolate breakdown and insect defense. Plant J 2006; 46:549-562; PMID:16640593; http://dx.doi.org/10.1111/j.1365-313X.2006.02716.x
- Li M, Sack FD. Myrosin idioblast cell fate and development are regulated by the Arabidospsis transcription factor FAMA, the auxin pathway, and vesicular trafficking. Plant Cell 2014; 26:4053-4066; PMID:25304201; http://dx.doi.org/10.1105/tpc.114.129726
- Shirakawa M, Ueda H, Nagano AJ, Shimada T, Kohchi T, Hara-Nishimura I. FAMA is an essential component for the differentiation of two distinct cell types, myrosin cells and guard cells, in Arabidopsis. Plant Cell 2014; 26:4039-4052; PMID:25304202; http://dx.doi.org/10.1105/tpc.114.129874
- Kang J, Dengler N. Vein pattern development in adult leaves of Arabidopsis Thaliana. Int J Plant Sci 2004; 165:231-242; http://dx.doi.org/10.1086/382794
- Scarpella E, Francis P, Berleth T. Stage-specific markers define early steps of procambium development in Arabidopsis leaves and correlate termination of vein formation with mesophyll differentiation. Development 2004; 131:3445-3455; PMID:15226260; http://dx.doi.org/10.1242/dev.01182
- Sawchuk MG, Donner TJ, Head P, Scarpella E. Unique and overlapping expression patterns among members of photosynthesis-associated nuclear gene families in Arabidopsis. Plant Physiol 2008; 148:1908-1924; PMID: 18820083; http://dx.doi.org/10.1104/pp.108.126946
- Shirakawa M, Ueda H, Koumoto Y, Fuji K, Nishiyama C, Kohchi T, Hara-Nishimura I, Shimada T. Continuous Vascular Ring (COV1) is a trans-golgi network-localized membrane protein required for golgi morphology and vacuolar protein sorting. Plant Cell Physiol 2014; 55:764-772; PMID:24363287; http://dx.doi.org/10.1093/pcp/pct195
- Sawchuk MG, Head P, Donner TJ, Scarpella E. Time-lapse imaging of Arabidopsis leaf development shows dynamic patterns of procambium formation. New Phytol 2007; 176:560-571; PMID:17953541; http://dx.doi.org/10.1111/j.1469-8137.2007.02193.x
- Shirakawa M, Ueda H, Shimada T, Kohchi T, Hara-Nishimura I. Myrosin cell development is regulated by endocytosis machinery and PIN1 polarity in leaf primordia of Arabidopsis thaliana. Plant Cell 2014; 26:4448-4461; PMID:25428982; http://dx.doi.org/10.1105/tpc.114.131441
- Donner TJ, Sherr I, Scarpella E. Regulation of preprocambial cell state acquisition by auxin signaling in Arabidopsis leaves. Development 2009; 136:3235-3246; PMID: 19710171; http://dx.doi.org/10.1242/dev.037028
- Shirakawa M, Ueda H, Shimada T, Nishiyama C, Hara-Nishimura I. Vacuolar SNAREs function in the formation of the leaf vascular network by regulating auxin distribution. Plant Cell Physiol 2009; 50:1319-1328; PMID:19493960; http://dx.doi.org/10.1093/pcp/pcp076
- Shirakawa M, Ueda H, Shimada T, Koumoto, Y, Shimada TL, Kondo M, Takahashi T, Okuyama Y, Nishimura M, Hara-Nishimura I. Arabidopsis Qa-SNARE SYP2 proteins localized to different subcellular regions function redundantly in vacuolar protein sorting and plant development. Plant J 2010; 64:924-935; PMID:21143674; http://dx.doi.org/10.1111/j.1365-313X.2010.04394.x
- Bergmann DC, Lukowitz W, Somerville CR. Stomatal development and pattern controlled by a MAPKK kinase. Science 2004; 304:1494-1497; PMID:15178800; http://dx.doi.org/10.1126/science.1096014
- Ohashi-Ito K, Bergmann DC. Arabidopsis FAMA controls the final proliferation/differentiation switch during stomatal development. Plant Cell 2006; 18:2493-2505;PMID:17088607; http://dx.doi.org/10.1105/tpc.106.046136
- MacAlister CA, Ohashi-Ito K, Bergmann DC. Transcription factor control of asymmetric cell divisions that establish the stomatal lineage. Nature 2007; 445:537-540; PMID:17183265; http://dx.doi.org/10.1038/nature05491
- Pillitteri LJ, Sloan DB, Bogenschutz NL, Torii KU. Termination of asymmetric cell division and differentiation of stomata. Nature 2007; 445:501-505; PMID:17183267; http://dx.doi.org/10.1038/nature05467
- Pillitteri LJ, Dong J. Stomatal development in Arabidopsis. The Arabidopsis Book 2013; 11:e0162; PMID:23864836; http://dx.doi.org/10.1199/tab.0162
