ABSTRACT
Small interfering RNAs (siRNAs) are involved in the regulation of plant development and response to stress. We have previously shown that mutants impaired in Dicer-like 2 (DCL2), DCL3 and DCL4, RDR2, RDR6 and NPRD1 are partially impaired in their response to stress and dcl2 and dcl3 plants are also impaired in transgenerational response to stress, including changes in homologous recombination frequency (HRF). Here, we have analyzed genome stability of dcl2, dcl3, dcl4, dcl2 dcl3, dcl2 dcl3 dcl4 and rdr6 mutants by measuring the non-induced and the stress-induced recombination frequency. We found that all mutants had the lower spontaneous HRF. The analysis of strand breaks showed that all tested Arabidopsis mutants had a higher level of spontaneous strand breaks, suggesting that the lower HRF is not due to the unusually low level of breaks. Exposure to methyl methane sulfonate (MMS) resulted in an increase in the level of strand breaks in wild-type plants and a decrease in mutants. All mutants had the higher methylation of cytosines at CpG sites under non-induced conditions. Exposure to MMS resulted in a decrease in methylation level in wild-type plants and an increase in methylation in all dcl mutants. The expression of several DNA repair genes was altered in dcl4 plants under non-induced and induced conditions. Our data suggest that siRNA biogenesis may be essential for the maintenance of the genome stability and stress response in Arabidopsis.
Introduction
Plants respond to stress at multiple levels, including the generation of free radicals, changes in transcriptome, the activation of protective mechanisms such as the production of osmolites, changes in membrane fluidity, the up-regulation of DNA damage repair processes as well as changes in other metabolic processes.Citation1,2 Many of the aforementioned mechanisms are the result of epigenetic changes in response to stress in the form of global genome- and loci-specific changes in DNA methylation, histone modifications, nuclear architecture and the activity of non-coding RNAs (ncRNAs).Citation3 The differential expression of ncRNAs is an essential part of stress response mechanisms. Among those are microRNAs (miRNAs) and various types of small interfering RNAs (siRNAs), including the naturally occurring siRNAs (nat-siRNAs), transactivating siRNAs (ta-siRNA), heterochromatic siRNAs (hc-siRNA) and long non-protein coding RNAs (npcRNA) among others.Citation4 miRNAs play an essential role in plant development and in response to stress.Citation5-8 siRNAs, on the other hand, are required to maintain silencing of repetitive and transposable elements in the genome, for cell protection against invading foreign nucleic acids (such as viruses and viroids) or aberrant endogenous RNAs,Citation9 they are also needed for the transcriptional regulation at the specific genic regions and for the posttranscriptional regulation in response to stress.Citation7 An additional role for siRNAs has recently been suggested. A special class of DSB-induced small RNAs (diRNAs) from the sequences around DSB sites has been identified and proposed to be involved in regulation of DNA repair and genome stability.Citation10,11
In the past, we found that siRNA biogenesis mutants were impaired in response to stress, DNA repairCitation12 and in the establishment of transgenerational memory of stress.Citation13 Analyzing stress response in mutants, we noticed that they had lower frequency of homologous recombination (HRF). Homologous recombination (HR) is a relatively error-free repair of strand breaks that utilizes homologous chromosomes or sister chromatids as a repair template.Citation14 HR is the mechanism involved in crossing-over and thus in genome rearrangements and genome diversification. Therefore, the analysis of frequency of occurrence of HR may reflect genome stability of a given plant. In this work, we analyzed HRF in wild-type plants and siRNA biogenesis mutants, dcl2, dcl3, dcl4, dcl2 dcl3, dcl2 dcl3 dcl4 and rdr6 plants using transgenic line 15d8.Citation15 This line carries in its genome a single copy of a luciferase recombination substrate that consists of 2 overlapping non-functional copies of the gene cloned in a direct orientation. These plants allow the analysis of recombination between homologous repeats within a transgenic locus as well as between sister chromatids Citation15; these plants have been used for the analysis of HRF in response to many different stresses.Citation13,16-18
We found that all the tested mutants had lower spontaneous HRF and also a lower increase in HRF in response to stress as compared to wild-type plants. Mutants also had a higher level of strand breaks as well as higher levels of methylation of cytosines under non-induced conditions. Treatment with methyl methane sulfonate (MMS) changed the levels of methylation and strand breaks, and again these changes were different in mutants. Our work suggests that DCL proteins may be necessary for the maintenance of genome stability under non-induced and induced conditions.
Materials and methods
Plant lines and growth conditions
The transgenic line #15d8 was used in the experiments. This line contains a single transgene copy consisting of 2 truncated overlapping parts of the luciferase gene cloned in a direct orientation.Citation19 The homologous recombination between 2 regions of homology within the same locus or between sister chromatids results in the restoration of an active luciferase gene. The following Arabidopsis thaliana mutant lines were crossed to #15d8 line: dcl2-5 [in Col-0; Citation20], dcl3-1 [in Col-0; Citation21], dcl4-2 [in Col-0; Citation22], rdr6-15 [in Col-0; Citation23], d2d3 [Col-0; Citation24], d2d4 [Col-0; Citation24], d2d3d4 [Col-0; Citation24]. Plants homozygous for the recombination substrate and mutations of the aforementioned gene(s) (as determined by gene-specific or transgene-specific PCRs) were used for further experiments.Citation13
Seeds were sown onto all-purpose soil with 25% vermiculite and kept at 4°C for 48 h to break dormancy. After germination, plants were transplanted at a density of 4 plantlets per a 10×10 cm pot. All plants were grown in soil at 22°C/ 18°C under a 12 h/12 h day/night photoperiod and illumination by 100 µM m−2 sec−1 during the day time. To establish drought conditions watering was stopped at day 7 and resumed at day 30 post germination to obtain seeds. Plants were exposed to flood by making sure that the pots were standing in water all the time. For heat exposure, plants were exposed to 42°C for 2 h/day for one week starting at 7 d post-germination. For cold exposure, plants were exposed to 4°C for 12 hours during the night for one week starting at 7 d post-germination. For UVC exposure, plants received 1.2 J m−2 as a single acute dose (0.01 J m−2 sec−1); exposure to UVC was done at 14 d post-germination. After exposure, plants were grown under normal conditions (see above). None of the stress conditions resulted in changes in plant appearance. In all cases, the homologous recombination frequency was analyzed at 30 d post-germination.
To expose plants to methyl methane sulfonate (MMS), plants were germinated on half Murashige and Skoog medium; at the age of one week, plants were transferred to normal MS medium or MS medium supplemented with 80 ppm MMS. Tissues were collected 2 weeks after transplanting. All experiments were repeated 3 times.
Analysis of HRF using a luciferase camera
The frequency of homologous recombination was analyzed using a luciferase CCD camera by counting luciferase sectors after spraying with luciferin.Citation19 HRF was calculated by relating the number of recombination events in the entire population of plants to the total number of plants used.
An average HRF value (with SD) was calculated 30 d post-germination using 3–5 independent experiments. In each experiment, 3 different pots (consisting of 4 small 10×10 cm pots) were used (16 plants in each of them).
The random oligonucleotide primed synthesis assay
The ROPS assay was performed as described before.Citation25 The total genomic DNA was also prepared as described.Citation25 The ROPS assay is based on the ability of the Klenow fragment polymerase (New England Biolabs) to initiate the random oligonucleotide-primed synthesis from the re-annealed 3′ OH ends of single-stranded (ss)DNA. The ssDNA formed upon a denaturation-reassociation step serves as its own primer by randomly reassociating itself to other ssDNA molecules. The incorporation of [3H]-dCPT into a newly synthesized DNA is proportional to the initial number of 3′OH ends (breaks).Citation26 The experiment was performed 2 times, with 2 independent measurements per each experiment. The average (with SD) [3H]-dCPT incorporation was calculated from 4 measurements.
Semi-quantitative Real Time PCR analysis
Approximately 100 mg of plant tissue was used for RNA extraction. Tissue was ground in liquid nitrogen and then transferred to a pre-chilled 1.5 mL Eppendorf tube. Next, 160 µL TRIzol reagent (Invitrogen) was added into the powder. The remainder of the extraction was performed as per the manufacturer's protocol. Quantity and quality of the RNA were measured using a spectrophotometer (Ultraspec 1100 pro). cDNA was then prepared from total RNA using the RevertAID H first strand cDNA synthesis kit (Fermentas).
Semi-quantitative Real Time PCR was performed in a total volume of 25 µL using 1 µL of cDNA template, 300 nM forward primer (see a full list of primers in ). First, a saturation curve was analyzed using different amounts of templates and different numbers of cycles. The conditions that did not result in saturation of PCR reactions were used. The selected cDNAs were amplified under the following conditions: (i) at 95°C for 5 min for one cycle; (ii) at 94°C for 30s, 56.8°C (see for temperatures for each specific primer) for 30s, at 72°C for 30s for 18 25 cycles (tubulin – 18 cycles; ligase 1, AtFPG1 and Polλ – 24 cycles; Rad51, KU70, MSH2 and MSH7 – all 25 cycles); and (iii) at 72°C for 10 min for one cycle. Each experiment was repeated in triplicates. Quantifications of band intensity were made using the program ImageQuant Version 5.2. The statistical significance of the experiment was confirmed by performing a Student's t-test (2-tailed paired or unpaired).
Table 1. The sequence of primers used in the experiments and the melting temperatures (Tm).
The comet assay
For the comet assay, protoplasts were isolated from 3-week-old treated and non-treated wt and mutant plants. Protoplast isolation was done using the Tape-Arabidopsis Sandwich protocol as described before.Citation17 A prepared protoplast solution was kept on ice until further required for the comet assay analysis. The neutral comet assay was performed as previously published.Citation27 The comet slides were stained with SYBR Gold Nucleic Acid Gel Stain for 20 min and viewed in epifluorescence with a Zeiss Observer.Z1 microscope using a Stingray CCD camera (Allied Vision Technologies). The length of comet tails was evaluated with the Comet Assay IV software system (Perceptive Instruments Ltd.). Two independent biological replicas with 2 technical repeats were performed. Over 100 comets per experimental group were scored, and the average (with SD) from 4 measurements was calculated.
The analysis of global genome methylation using the cytosine extension assay
For the analysis of global genome methylation, genomic DNA was prepared from tissues of 3-week- old non-treated and MMS-treated control and dcl2, dcl3, dcl4 and rdr6 mutant plants. In each case, 10 plantlets were taken for the extraction of DNA using Trizol reagent as published before.Citation16
For the analysis, 3 µg DNA from each sample was digested for 48 h using a 10-fold excess of either HpaII or MspI endonuclease as per the manufacturer's protocol (New England Biolabs, Beverly, MA). To have a background control, a separate DNA aliquot was used without any restriction enzyme. After digestion, a single nucleotide extension reaction was performed in 2 µg of DNA using the cytosine extension assay as described before.Citation16 The analysis was done 4 times from 2 biological and 2 technical repeats. The radioactive count of the extension reaction was presented in dpm/µg of DNA. The average of 4 measurements was calculated after subtracting readings from background controls. The level of methylation correlates inversely with radioactive counts – the higher the counts, the more DNA was digested with methylation-sensitive enzymes, thus the lower the level of methylation. Therefore, to calculate the percentage increase or decrease in methylation levels, we used the following formula: methylation = 100/X*100, where X is the radioactive count for a particular sample.
Statistical analysis
The experiments were repeated 3 to 5 times, and mean values with SD were calculated. The statistical significance of the results was confirmed by performing a Student's t-test. Statistical analyses were performed using Microcal Origin 6.0 software.
Results
Recombination frequency in siRNA biogenesis mutants is different from that in wild-type plants
To analyze homologous recombination frequency, we crossed Arabidopsis thaliana line 15d8 (Col-0 background) with the following mutants: dcl2, dcl3, dcl4, rdr6 as well as double and triple mutants, dcl2 dcl3 (d2d3 thereafter) and dcl2 dcl3 dcl4 (d2d3d4 or triple mutants thereafter). For the sake of simplicity, we will refer to 15d8 plants as wild-type plants (wt) and to single, double and triple dcl mutants as mutants.
The analysis of HRF () in homozygous mutants of the F4 progeny showed that all mutants had a lower recombination frequency, with the lowest HRF being in dcl2 and dcl3 plants; whereas wt plants had nearly 6 recombination events per plant, mutants had a significantly lower number of events, with dcl2 and dcl3 having as few as 1.2 1.4 events per plant (). In the mutants, there were many plants that had a single recombination event or no detectable events at all ().
Figure 1. HRF in wt and mutant plants (A) A schematic representation of the luciferase-based recombination substrate and a representative image of recombination events in wt plants. The LUC-based recombination substrate consists of 2 truncated copies of the LUC gene with partial overlapping regions. Recombination events between the regions of overlap (depicted as “U”) result in the restoration of the LUC transgene. Transgene activity can be monitored by spraying plants with luciferin and counting recombination events using a special CCD camera. An example of the CCD camera image is shown. (B) HRF in wt plants and dcl mutants. HRF (an average number of spots per plant with SD) was calculated from 5 independent experiments. The asterisks show the difference in HRF either between wt and mutant plants or between mutant plants (the asterisks over horizontal bars). One asterisks is p < 0.05, two – p < 0.01, three – p < 0.001
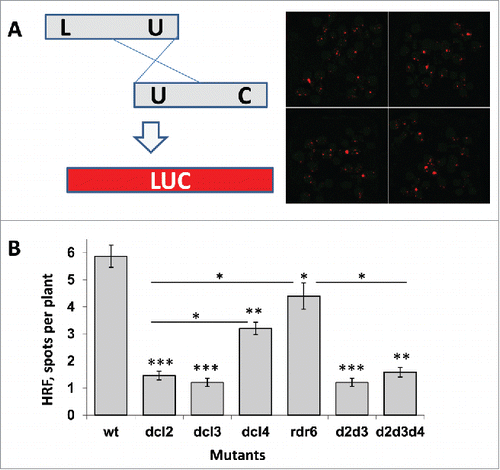
Figure 2. The distribution of recombination events in dcl2 (A), dcl3 (B), dcl4 (C), rdr6 (D), dcl2 dcl3 (E), dcl2 dcl3 dcl4 (F) plants in comparison to wt plants The Y-axis shows the number of plants, whereas the X-axis shows the number of events (spots) per single plant.
Stress exposure is known to increase genome instability reflected by changes (mostly an increase) in HRF. Since previous experiments demonstrated that dcl mutants had different sensitivity to stress compared to wild-type plants, we hypothesized that HRF in mutants would also change differently.
In mutants, HRF changed differently in response to stress
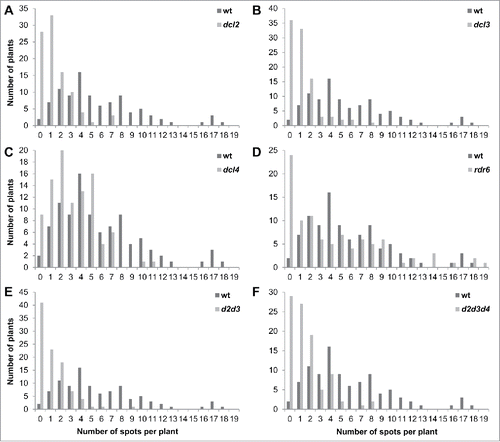
To test whether in mutants HRF changes differently in response to stress, we exposed plants to UVC, heat, cold, drought and flood. Mutants responded with different levels of changes as compared to wild- type plants (). In particular, in response to UVC, dcl2 and triple mutants responded with a higher increase in HRF. In response to cold, d2d3 plants had a lower increase in HRF, whereas in response to heat, dcl4 and triple mutants exhibited a higher increase in HRF and d2d3 had a lower increase. In response to flood, dcl2 and d2d3 had a lower increase, and triple mutants had a higher increase, whereas in response to drought, all mutants but dcl4 exhibited a higher HRF increase (). Curiously, in all cases, an increase in HRF in response to stress was higher in triple mutants than in wt plants, except in case of exposure to cold. Although an individual response of mutants to stress was different from that in wild-type plants, a single-factor ANOVA analysis showed that only in triple mutants, the response was significantly different compared to wild-type plants, as far as all stresses were concerned (p < 0.05).
Figure 3. Changes in HRF in response to UVC (A), cold (B), heat (C), flood (D) and drought (E). The Y-axis shows the average (with SDSD) changes in HRF in wt and mutant plants exposed to stress as compared to non-stressed plants. The asterisks show the difference in HRF changes between wt and mutant plants. One asterisks is p < 0.05, two – p < 0.01, three – p < 0.001.
Mutants have higher levels of spontaneous strand breaks
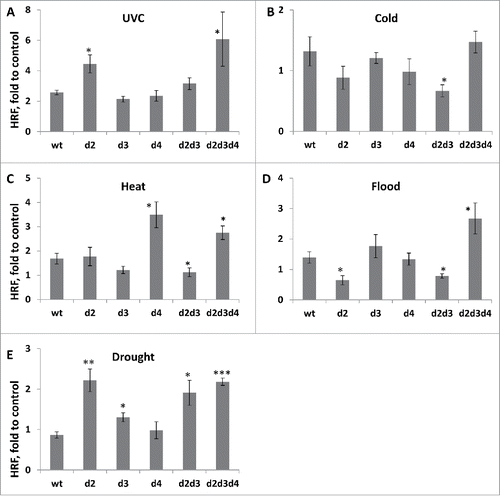
A lower HRF value in mutants could be due to a lower spontaneous level of strand breaks. To test this, we performed 2 assays: the neutral comet assay Citation28 and the random oligonucleotide primed synthesis (ROPS) assay.Citation29 The comet assay determines the comet tail moment upon single cell gel electrophoresis; the neutral comet assay allows to measure DSBs.Citation28 In contrast, the ROPS assay measures the incorporation of radioactively labeled nucleotides upon the repair of 3′ OH DNA breaks initiated by the Klenow fragment polymerase; the ROPS assay makes it possible to measure both, SSBs and DSBs. Both assays have shown that mutants have higher levels of breaks (). Thus, a lower value of HRF is not due to a lower level of strand breaks.
Figure 4. The level of strand breaks in wt and mutant plants SSBs and DSBs levels as measured using the ROPS assay. The data are shown as a percentage to wt (an average with SD), where wt is taken as 100%. The asterisks show a significant difference in the levels of strand breaks between wt and mutant plants. One asterisks is p < 0.05, two – p < 0.01. DSBs levels as measured using the comet assay. The data are shown as an average tail moment (with SD), and all the numbers are related to wt taken as 1. The asterisks (p < 0.01) show a significant difference in DSBs between wt and mutant plants. SSBs and DSBs levels in wt and mutant plants exposed to MMS as measured using the ROPS assay. The data are shown as a percentage to wt control samples (an average with SD), where wt is taken as 100%. The asterisks show a significant difference in the levels of strand breaks either between wt and mutant plants or between MMS treated and control plants (the asterisks over bars). One asterisks is p < 0.05, two – p < 0.01.
Next, we tested whether the level of strand breaks would increase further in mutants upon exposure to methyl methane sulfonate (MMS). Exposure to MMS resulted in an increase in strand breaks in wild- type plants as measured by ROPS. Although MMS does not directly produce strand breaks, the repair of stalled replication forks may lead to the formation of transient strand breaks.Citation30 Curiously, exposure to MMS did not result in an increase in strand breaks in mutants, except d2d3d4 plants (). In fact, dcl3, dcl4 and d2d3 plants exposed to MMS had a lower number of breaks than non-exposed plants.
Mutants are mostly similar to wt plants in the expression of DNA repair enzymes
Lower levels of HRF and higher levels of strand breaks in mutants could be caused by the inability of DNA repair enzymes to cope with higher levels of DNA damage. This could be due to either lower levels of the expression of DNA repair genes or lower levels of the activity of repair proteins. In our previous work, we showed that dcl mutants had a lower capacity of repair of induced DNA damage.Citation12 Here, we tested the expression levels of DNA repair genes involved in the repair of MMS-induced lesions.
DCL mutants do not substantially differ from wild-type plants in the expression of BER genes
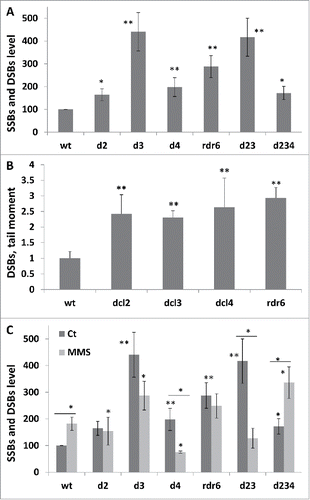
The analysis showed a similar non-induced steady-state RNA level of AtFPG1, polymeraseλ and DNA ligase 1 in wild-type and DCL mutants, except of ligase 1 in dcl2 and dcl4 mutants (). The expression of ligase 1 was significantly lower (P < 0.05) in dcl2 and dcl4. Exposure to MMS resulted in a substantial increase in the expression of all 3 g in all plants, but there was no significant difference between wt and mutants ().
Figure 5. The semi-quantitative RT-PCR (SQ-RTPCR) analysis of the expression of base excision repair, DSB repair and mismatch repaor genes in wt and mutant plants exposed to MMS The steady state RNA levels of AtFPG1 (A), ligase 1 (B), Polλ (C), Rad51 (D), KU70 (E), MSH2 (F) and MSH7 (G) are shown as an average of arbitrary units of intensity calculated from 2 biological and 2 technical repeats (with SD). The asterisks (p < 0.05) show a significant difference between mutants and wt plants.
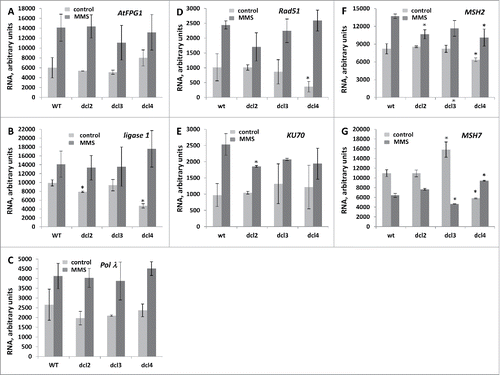
The expression of genes involved in DSB and MMR repair
The leftovers of BER repair are often taken care of by mismatch repair, polymerase bypass, and homologous recombination pathways.Citation31 Therefore, we decided to test the steady-state RNA levels of Rad51 and Ku70 (the enzymes involved in DSB repair) and Msh2 and Msh7 (the enzymes involved in mismatch repair). The analysis showed that despite substantial variations in the expression of the tested genes, only dcl3 and dcl4 mutants had a significantly different expression of repair enzymes under non-induced conditions as compared to wt; in dcl4, the level of RNA of Rad51, Msh2 and Msh7 genes was lower than in wt plants, whereas in dcl3, a higher expression of Msh7 gene was observed (). Moreover, in rdr6, the non-induced RNA levels of Rad51, Ku70 and Msh7 were also lower than in wt plants (data not shown). The induced expression of KU70 was lower in dcl2, the expression of Msh2 was lower in dcl2 and dcl4, and finally, Msh7 was lower in dcl3 and higher in dcl4 ().
Changes in DNA methylation
Although it has not been clearly established, there is a general “belief” that DNA methylation inversely correlates with genome stability and with HRF in particular. In fungi, the hypermethylation of the Ascobolus immerses spore color gene b2 results in several hundredfold reduction of the frequency of crossing-over.Citation32 In plants, the data are less conclusive. A recent work, however, showed that the loss of methylation resulted in an increase in the frequency of crossing-over events in the euchromatic regions and a decrease in the heterochromatic regions.Citation33,34
In plants, symmetrical CpG and CpNpG sites are the most common sites of methylation. To test global genome methylation, we used the methylation-sensitive HpaII and MspI restriction enzymes that can recognize the CCGG nucleotides. Methylation of the external cytosine in CCGG representing CNG methylation prevents the digestion with MspI and severely impairs the digestion with HpaII.Citation35 Methylation of the internal cytosine in CCGG representing CG methylation does not influence the digestion with MspI but prevents the digestion with HpaII. Thus, the digestion with MspI allows to evaluate the difference in methylation at symmetrical CNG sites between wt and mutant plants.
Our analysis showed that methylation levels as measured using the HpaII restriction enzyme were higher in all non-exposed mutants (). In contrast, the analysis of methylation using the MspI enzyme showed lower methylation levels in non-exposed tissues of dcl3 plants and higher methylation levels in rdr6 plants.
Figure 6. DNA methylation levels in wt and mutant plants in response to MMS. Global genome methylation was analyzed using the cytosine extension assay. Genomic DNA was digested with methylation-sensitive enzymes, HpaII (A) and MspI (B), and the average 3H incorporation (in dpm/μg) was calculated from 4 biological and 2 technical repeats. DNA methylation was calculated using the following formula: methylation = 100/X*100, where X is the radioactive count for a particular sample. DNA methylation is shown in a percentage to wt control plants (wt ct), where wt ct is taken as 100%. The asterisks show a significant difference between mutant and wt samples or between treatment and control samples, where one asterisk is p < 0.05, two – p < 0.01 and three – p < 0.001.
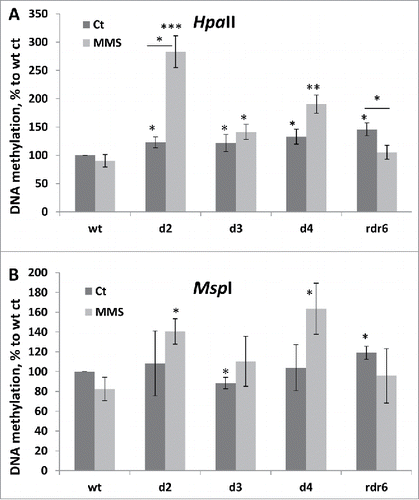
Exposure to MMS resulted in a decrease in global genome methylation in wild-type plants, as shown using HpaII and MspI restriction digestion. Global genome hypomethylation is a common response of somatic cells to stress.Citation36,37 The response was more pronounced upon MspI digestion, suggesting that demethylation in response to stress is more pronounced at CNG sites. In the mutants, methylation increased in dcl mutants but decreased in rdr6 mutant. Methylation was significantly higher in all dcl mutants in the exposed tissues, as shown using HpaII enzyme and dcl2 and dcl4 plants using MspI enzyme ().
Discussion
Double-strand breaks are one of the most deleterious DNA lesions. They are repaired through two 2 main mechanisms, non-homologous end joining (NHEJ) and homologous recombination (HR), each having several partially overlapping pathways.Citation38 Whereas the former is more frequent but less precise, the latter one is less frequent but a more precise repair mechanism. In addition, the balance between NHEJ and HR repair pathways is finely regulated at different stages of the cell cycle; HR seems to be suppressed in G1 phase where it can lead to homozygosity for a deleterious mutation, but it is more active in S/G2 phases when sister chromatids are present.Citation39
Various stresses were shown to cause DNA damage and lead to an increase in HRF, whether directly or indirectly.Citation40-44 Since stresses such as exposure to heat, cold or salt are not damaging DNA directly, we hypothesized that an increase in HRF in response to these stresses is in part due to non-targeted effects that could be caused by the activity of small non-coding RNAs. We have shown that Dicer-like mutants dcl2 and dcl3 are impaired in an increase in HRF in the progeny of stressed plants.Citation13 Moreover, we have shown that dcl mutants are impaired in response to stress and in the DNA repair capacity.Citation12
Our current work has demonstrated that siRNA biogenesis mutants have a lower frequency of spontaneous homologous recombination. Also, changes in HRF in response to stress are also different in mutants as compared to wild-type plants. Lower HRF values can be due to a number of different reasons. First of all, it is possible that siRNA biogenesis mutants have lower endogenous levels of strand breaks; but our analysis showed that this was not actually the case – levels of strand breaks were elevated in mutants. This suggests that mutants have either a higher frequency of strand break occurrence or a lower level of repair of such breaks. DNA strand breaks may occur due to a number of reasons, with a higher metabolic activity and an increased formation of free radicals being some of them. In a single cell, 1% of SSBs is converted to DSBs, resulting in ˜50 DSBs per cell per cell cycle.Citation45 Dicer-like mutants do not appear to have an accelerated cell growth, and they look phenotypically similar to wild-type plants, therefore it is unlikely that they have an increased metabolic rate or a higher production of free radicals, although this needs to be tested.
It is unfortunately very difficult to measure the level of spontaneous strand breaks because most of the methods, including both used in this work, show a snapshot picture at the moment of DNA extraction. They also show the levels of strand breaks that have just occurred and those ones that have occurred earlier but have not been repaired. Therefore, it is hard to be certain whether strand breaks analyzed in this work are naturally more frequent in mutants or they are just poorly repaired; it is likely that it is both. Our previous work demonstrated that siRNA biogenesis mutants are indeed impaired in the repair of damaged DNA.Citation12
Two recent key publications also bring the evidence that siRNA biogenesis mutants are likely impaired in DSB repair. Using the transgenic Arabidopsis system that allows to induce strand breaks by the expression of a rare-cutting enzyme, reports showed that DSBs trigger the production of a special class of DSB-induced small RNAs (diRNAs) from the sequences around DSB sites.Citation10,11 In plants, diRNAs may play a dual role; firstly, diRNAs may guide certain histone modifications at the site of DNA damage; secondly, diRNAs may play a more direct role in DSB repair by recruiting DSB repair complexes to DNA lesions using the effector protein AGO2.Citation10 Furthermore, in humans, it has been demonstrated that Ago2 is likely directing Rad51 accumulation at the DSB sites; this interaction is enhanced in the irradiated tissues.Citation11 Interestingly, it has been shown that diRNAs are not necessary for the interaction of Ago2 with Rad51 but rather for the formation of Rad51 foci and the efficiency of HR.Citation11 The depletion of Dicer in human cells has been shown to affect the efficiency of DBS repair and the accumulation of Rad51 at the repair site.Citation10 Curiously, diRNAs seem to be involved specifically in the HR pathway of DSB repair rather than in the NHEJ pathway; the depletion of Ago2 in Dicer mutants does not decrease the frequency of NHEJ repair.Citation11
It is likely that the decreased HRF that we have found in this work is the reflection of lower levels of diRNAs formed against the transgenic recombination locus. Indeed, Wei et al. (2012) revealed that in the dcl3 mutant, the number of diRNAs was reduced by 98%, whereas in the rdr2 and rdr6 mutants, the number of diRNAs was reduced by 87% and 82%, respectively. Previous work by Wei et al. (2012) has reported a decrease in HRF in the dcl2, dcl3, and dcl4 mutants by 42%, 90%, and 44%, respectively, compared to that in the wild-type plants.Citation10 Our data are similar: there has been a decrease in the rate of spontaneous HRF in the dcl2, dcl3 and dcl4 mutants by ∼75%, 80% and 40%, respectively. The difference may be attributed to the fact that the structure of our recombination construct was different, and we did not use the induction by a restriction enzyme. A similar reduction in spontaneous HRF was found in the atrad51C mutant; there was a 2-fold decrease in HRF in the mutant as compared to wt plants.Citation46 Also, the bleomycin- and cisplatin-induced HRF was also impaired in the atrad51C mutant.
In this work, in most of the cases, the expression of DNA repair genes was unchanged, except for DNA ligase 1 in the dcl2 and dcl4 mutants and Rad51, MSH2 and MSH7 in the dcl4 mutant, where it was lower than in wt plants. Similarly, Wei et al. (2012) showed that Rad51 expression was also unchanged in the dcl3 mutant.Citation10 Since mutants had higher levels of strand breaks, it should be expected that they would have a higher rate of HRF. So, it is possible that a lower rate of HRF observed in our work and in the work of Wei et al. (2012) is due to a more condensed chromatin in these mutants, at least at the site of transgene integration. Condensed chromatin partly correlates with high methylation levels; indeed, we found that methylation levels at CG sites were higher in mutants (except dcl4).
Our work shows that Dcl mutants are altered in DNA methylation. Previously, a comprehensive analysis of DNA methylation using bisulfite sequencing showed that global genome methylation in dcl2 and dcl4 mutants was similar to that in wt plants, whereas in dcl3 mutant, it was slightly lower.Citation47 Also, the level of methylation in triple mutants was substantially lower than in wt plants. Also, another work that used bisulfite sequencing method demonstrated that methylation in the Tag2 transposon-related sequence and 5S rDNA repeats was reduced in dcl3 mutant.Citation48 These data are different (in part) from our finding. For example for dcl3 mutant, our analysis also showed that the level of methylation in dcl3 was decreased when MspI was used, but it was actually increased when HpaII was used. The level of methylation at TAS3a locus was decreased in dcl1, but it was not decreased in dcl3 and dcl4 mutants.Citation49 Curiously, methylation at TAS1c locus was increased in dcl4 mutant and decreased in dcl1 mutant. Also, the level of methylation at TAS3a and TAS1c loci was slightly increased in dcl2/dcl4 double mutant.Citation49 We also observed an increase in global genome DNA methylation in dcl4. Another most recently published paper reported analysis of DNA methylation in RdDM targeted DNA loci and showed that they were not decreased in DNA methylation in dicer mutants.Citation50 All in all, our data on DNA methylation differ from the previous findings. The discrepancy could come from the fact that we have analyzed methylation at specific CCGG sites that are present mainly around genic regions and occur more commonly in the promoter area. Also, the data mentioned above (except Stroud et al. (2013) Citation47 measured methylation in specific genomic loci, whereas we have done it globally in the whole genome. Also, all the above mentioned reports utilized bisulfite conversion method, whereas in our case, we used methylation sensitive enzymes method. It would be interesting to perform direct comparisons using these 2 methods. It also remains to be demonstrated whether methylation is more different in genic versus intergenic regions in dcl mutants compared to wild-type plants.
We have found an increase in methylation in dcl mutants in response to MMS. MMS is a DNA-methylating agent that generates 7-methylguanine (N7-MeG) in over 80% of cases.Citation31,51 MMS does not directly induce strand breaks, but rather through the generation of BER strand break intermediates.Citation31 Treatment with methyl methane sulfonate may have interfered with DNA methylation analysis, although we could not find any information suggesting that guanine methylation (N7-MeG generated by MMS) at the CCGG site interferes with digestion by either HpaII or MspI enzyme. If this was the case, we would have observed an increase in DNA methylation in response to MMS in wild- type plants, whereas we actually have observed a decrease.
Changes in the genome stability and in the frequency of homologous recombination observed in Dcl mutants are likely not only due to the decreased levels of diRNAs produced against the luciferase transgene locus. Since biogenesis of many siRNAs are impaired in mutants, even so Dicers are known to substitute each other's function, it is possible that the expression of some key regulators of chromatin structure, DNA damage recognition, or DNA repair genes (except those that have been tested in this work) is impaired, especially in response to stress. siRNAs target loci in a sequence-specific manner in response to various environmental cues. For example, in response to low humidity, genes that control stomatal development are methylated by sequence-specific siRNAs.Citation52 It remains to be shown whether changes in chromatin structure and methylation of the above-mentioned loci contribute to a lower frequency of recombination and a deficiency of siRNA mutants in DNA repair.
It is important to note that the findings described here and conclusions from them are made from studying Arabidopsis mutants. Limited work is published in respect of Dcl mutants in other plants. Although our findings might be restricted to Arabidopsis only, a plant with a small duplicated genome, several other papers reported the similarity of findings for Arabidopsis and rice Dcl mutants. In rice, one of the Arabidopsis DCL3 homologs, OsDCL3a, regulates the production of transposon-derived siRNAs,Citation53 a function similar to the one maintained by Arabidopsis DCL3. Similarly, the DCL4 homolog OsDCL4 is involved in biogenesis of ta-siRNAs and phased small non-coding RNAs Citation54; again, this function is similar to that of DCL4 in Arabidopsis.Citation55 It remains to be shown whether DCL proteins play a similar role in the regulation of genome stability in other dicots and monocots, especially in those ones with large complex genomes, such as in wheat.
Conclusion
Our work has contributed to the understanding of the role of siRNA and siRNA biogenesis proteins in the maintenance of genome stability and specifically in the repair of strand breaks by homologous recombination. Low HRF is likely due to 2 factors: higher methylation levels and a lower amount of diRNAs formed against the transgenic loci. The absence of certain siRNAs and the lack of proper methylation/demethylation at the specific genomic loci may be sufficient to trigger the differential genome stability and a lower HRF in siRNA biogenesis mutants. It is however unclear to what degree siRNAs or other small and long ncRNAs regulate DNA damage repair or DNA damage mitigation. Our data and data of Wei et al. (2012) have demonstrated a lower HRF at transgenic loci. Since siRNAs are involved in the regulation of chromatin structure of transgenic loci and transgenes are at a higher scrutiny than endogens with a similar DNA sequence,Citation56 it is possible that the effect that siRNAs have on genome stability of transgenic loci is different from that in endogenous loci. It still remains to be shown whether HR is impaired at endogens in the siRNA biogenesis mutants.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We acknowledge the financial support of Alberta Innovates Biosolutions and Natural Science and Engineering Research Council of Canada grants to Igor Kovalchuk and Alberta Innovates Technology Features for scholarship to Andriy Bilichak. We thank Valentina Titova for proofreading the manuscript.
Author contribution
YY – performed crosses between rdr6 mutant and recombination line, performed all stress exposures, measured HRF and analyzed data. AB analyzed strand breaks and methylation levels in normal and exposed conditions. AG performed real time PCR analysis of DNA repair genes and analyzed data. IK designed the experiments, analyzed data and wrote the manuscript.
References
- Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front Plant Sci 2014; 5:170; PMID:24904597; http://dx.doi.org/10.3389/fpls.2014.00170
- Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R. Abiotic and biotic stress combinations. New Phytol 2014; 203:32-43; PMID:24720847; http://dx.doi.org/10.1111/nph.12797
- Vriet C, Hennig L, Laloi C. Stress-induced chromatin changes in plants: of memories, metabolites and crop improvement. Cell Mol Life Sci 2015; PMID:25578097; http://dx.doi.org/10.1007/s00018-014-1792-z
- Matsui A, Nguyen AH, Nakaminami K, Seki M. Arabidopsis non-coding RNA regulation in abiotic stress responses. Int J Mol Sci 2013; 14:22642-54; PMID:24252906; http://dx.doi.org/10.3390/ijms141122642
- Li WX, Oono Y, Zhu J, He XJ, Wu JM, Iida K, Lu XY, Cui X, Jin H, Zhu JK. The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. The Plant Cell 2008; 20:2238-51; PMID:18682547; http://dx.doi.org/10.1105/tpc.108.059444
- Liu HH, Tian X, Li YJ, Wu CA, Zheng CC. Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. Rna 2008; 14:836-43; PMID:18356539; http://dx.doi.org/10.1261/rna.895308
- Matsui A, Mizunashi K, Tanaka M, Kaminuma E, Nguyen AH, Nakajima M, Kim JM, Nguyen DV, Toyoda T, Seki M. tasiRNA-ARF pathway moderates floral architecture in arabidopsis plants subjected to drought stress. Bio Med Research Int 2014; 2014:303451; PMID:25243128; http://dx.doi.org/10.1155/2014/303451
- Xia J, Zeng C, Chen Z, Zhang K, Chen X, Zhou Y, Song S, Lu C, Yang R, Yang Z, et al. Endogenous small-noncoding RNAs and their roles in chilling response and stress acclimation in Cassava. BMC Genomics 2014; 15:634; PMID:25070534; http://dx.doi.org/10.1186/1471-2164-15-634
- Seo JK, Wu J, Lii Y, Li Y, Jin H. Contribution of small RNA pathway components in plant immunity. Mol Plant-Microbe Interact 2013; 26:617-25; PMID:23489060; http://dx.doi.org/10.1094/MPMI-10-12-0255-IA
- Wei W, Ba Z, Gao M, Wu Y, Ma Y, Amiard S, White CI, Rendtlew Danielsen JM, Yang YG, Qi Y. A role for small RNAs in DNA double-strand break repair. Cell 2012; 149:101-12; PMID:22445173; http://dx.doi.org/10.1016/j.cell.2012.03.002
- Gao M, Wei W, Li MM, Wu YS, Ba Z, Jin KX, Li MM, Liao YQ, Adhikari S, Chong Z, et al. Ago2 facilitates Rad51 recruitment and DNA double-strand break repair by homologous recombination. Cell Res 2014; 24:532-41; PMID:24662483; http://dx.doi.org/10.1038/cr.2014.36
- Yao Y, Bilichak A, Golubov A, Blevins T, Kovalchuk I. Differential sensitivity of Arabidopsis siRNA biogenesis mutants to genotoxic stress. Plant Cell Rep 2010; 29:1401-10; PMID:20953786; http://dx.doi.org/10.1007/s00299-010-0930-9
- Boyko A, Blevins T, Yao Y, Golubov A, Bilichak A, Ilnytskyy Y, Hollunder J, Meins F Jr, Kovalchuk I. Transgenerational adaptation of Arabidopsis to stress requires DNA methylation and the function of Dicer-like proteins. PloS one 2010; 5:e9514; PMID:20209086; http://dx.doi.org/10.1371/journal.pone.0009514
- Knoll A, Fauser F, Puchta H. DNA recombination in somatic plant cells: mechanisms and evolutionary consequences. Chromosome Res 2014; 22:191-201; PMID:24788060; http://dx.doi.org/10.1007/s10577-014-9415-y
- Boyko A, Filkowski J, Kovalchuk I. Homologous recombination in plants is temperature and day-length dependent. Mutat Res 2005; 572:73-83; PMID:15790491; http://dx.doi.org/10.1016/j.mrfmmm.2004.12.011
- Boyko A, Kathiria P, Zemp FJ, Yao Y, Pogribny I, Kovalchuk I. Transgenerational changes in the genome stability and methylation in pathogen-infected plants: (virus-induced plant genome instability). Nucl Acids Res 2007; 35:1714-25; PMID:17311811; http://dx.doi.org/10.1093/nar/gkm029
- Yao Y, Bilichak A, Titov V, Golubov A, Kovalchuk I. Genome stability of Arabidopsis atm, ku80 and rad51b mutants: somatic and transgenerational responses to stress. Plant Cell Physiol 2013; 54:982-9; PMID:23574700; http://dx.doi.org/10.1093/pcp/pct051
- Bilichak A, Yao Y, Kovalchuk I. Transient down-regulation of the RNA silencing machinery increases efficiency of Agrobacterium-mediated transformation of Arabidopsis. Plant Biotechnol J 2014; 12:590-600; PMID:24472037; http://dx.doi.org/10.1111/pbi.12165
- Boyko A, Zemp F, Filkowski J, Kovalchuk I. Double-strand break repair in plants is developmentally regulated. Plant Physiol 2006; 141:488-97; PMID:16474027; http://dx.doi.org/10.1104/pp.105.074658
- Akbergenov R, Si-Ammour A, Blevins T, Amin I, Kutter C, Vanderschuren H, Zhang P, Gruissem W, Meins F Jr, Hohn T, et al. Molecular characterization of geminivirus-derived small RNAs in different plant species. Nucleic Acids Res 2006; 34:462-71; PMID:16421273; http://dx.doi.org/10.1093/nar/gkj447
- Xie Z, Johansen LK, Gustafson AM, Kasschau KD, Lellis AD, Zilberman D, Jacobsen SE, Carrington JC. Genetic and functional diversification of small RNA pathways in plants. PLoS Biol 2004; 2:E104; PMID:15024409; http://dx.doi.org/10.1371/journal.pbio.0020104
- Xie Z, Allen E, Wilken A, Carrington JC. DICER-LIKE 4 functions in trans-acting small interfering RNA biogenesis and vegetative phase change in Arabidopsis thaliana. Proc Natl Acad Sci U S A 2005; 102:12984-9; PMID:16129836; http://dx.doi.org/10.1073/pnas.0506426102
- Allen E, Xie Z, Gustafson AM, Carrington JC. microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 2005; 121:207-21; PMID:15851028; http://dx.doi.org/10.1016/j.cell.2005.04.004
- Blevins T, Rajeswaran R, Shivaprasad PV, Beknazariants D, Si-Ammour A, Park HS, Vazquez F, Robertson D, Meins F Jr, Hohn T, et al. Four plant Dicers mediate viral small RNA biogenesis and DNA virus induced silencing. Nucleic Acids Res 2006; 34:6233-46; PMID:17090584; http://dx.doi.org/10.1093/nar/gkl886
- Yao Y, Bilichak A, Golubov A, Kovalchuk I. Local infection with oilseed rape mosaic virus promotes genetic rearrangements in systemic Arabidopsis tissue. Mutat Res 2011; 709-710:7-14; PMID:21376739; http://dx.doi.org/10.1016/j.mrfmmm.2011.02.014
- Basnakian AG, James SJ. Quantification of 3′OH DNA breaks by random oligonucleotide-primed synthesis (ROPS) assay. DNA Cell Biol 1996; 15:255-62; PMID:8634154; http://dx.doi.org/10.1089/dna.1996.15.255
- Kozak J, West CE, White C, da Costa-Nunes JA, Angelis KJ. Rapid repair of DNA double strand breaks in Arabidopsis thaliana is dependent on proteins involved in chromosome structure maintenance. DNA Repair 2009; 8:413-9; PMID:19070688; http://dx.doi.org/10.1016/j.dnarep.2008.11.012
- Collins AR. The comet assay for DNA damage and repair: principles, applications, and limitations. Mol Biotechnol 2004; 26:249-61; PMID:15004294; http://dx.doi.org/10.1385/MB:26:3:249
- Golubov A, Yao Y, Maheshwari P, Bilichak A, Boyko A, Belzile F, Kovalchuk I. Microsatellite instability in Arabidopsis increases with plant development. Plant Physiol 2010; 154:1415-27; PMID:20817752; http://dx.doi.org/10.1104/pp.110.162933
- Lundin C, North M, Erixon K, Walters K, Jenssen D, Goldman AS, Helleday T. Methyl methanesulfonate (MMS) produces heat-labile DNA damage but no detectable in vivo DNA double-strand breaks. Nucleic acids research 2005; 33:3799-811; PMID:16009812; http://dx.doi.org/10.1093/nar/gki681
- Wyatt MD, Pittman DL. Methylating agents and DNA repair responses: Methylated bases and sources of strand breaks. Chem Res Toxicol 2006; 19:1580-94; PMID:17173371; http://dx.doi.org/10.1021/tx060164e
- Maloisel L, Rossignol JL. Suppression of crossing-over by DNA methylation in Ascobolus. Genes & Dev 1998; 12:1381-9; PMID:9573054; http://dx.doi.org/10.1101/gad.12.9.1381
- Mirouze M, Lieberman-Lazarovich M, Aversano R, Bucher E, Nicolet J, Reinders J, Paszkowski J. Loss of DNA methylation affects the recombination landscape in Arabidopsis. Proc Natl Acad Sci U S A 2012; 109:5880-5; PMID:22451936; http://dx.doi.org/10.1073/pnas.1120841109
- Melamed-Bessudo C, Levy AA. Deficiency in DNA methylation increases meiotic crossover rates in euchromatic but not in heterochromatic regions in Arabidopsis. Proc Natl Acad Sci U S A 2012; 109:E981-8; PMID:22460791; http://dx.doi.org/10.1073/pnas.1120742109
- McClelland M, Nelson M, Raschke E. Effect of site-specific modification on restriction endonucleases and DNA modification methyltransferases. Nucl Acid Res 1994; 22:3640-59; PMID:7937074; http://dx.doi.org/10.1093/nar/22.17.3640
- Wada Y, Miyamoto K, Kusano T, Sano H. Association between up-regulation of stress-responsive genes and hypomethylation of genomic DNA in tobacco plants. Mol Gen Genom 2004; 271:658-66; PMID:15148604; http://dx.doi.org/10.1007/s00438-004-1018-4
- Karan R, DeLeon T, Biradar H, Subudhi PK. Salt stress induced variation in DNA methylation pattern and its influence on gene expression in contrasting rice genotypes. PloS One 2012; 7:e40203; PMID:22761959; http://dx.doi.org/10.1371/journal.pone.0040203
- Mannuss A, Dukowic-Schulze S, Suer S, Hartung F, Pacher M, Puchta H. RAD5A, RECQ4A, and MUS81 have specific functions in homologous recombination and define different pathways of DNA repair in Arabidopsis thaliana. Plant Cell 2010; 22:3318-30; PMID:20971895; http://dx.doi.org/10.1105/tpc.110.078568
- Rothkamm K, Kruger I, Thompson LH, Lobrich M. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol Cell Biol 2003; 23:5706-15; PMID:12897142; http://dx.doi.org/10.1128/MCB.23.16.5706-5715.2003
- Richter KS, Kleinow T, Jeske H. Somatic homologous recombination in plants is promoted by a geminivirus in a tissue-selective manner. Virology 2014; 452-453:287-96; PMID:24606706; http://dx.doi.org/10.1016/j.virol.2014.01.024
- Li F, Liu P, Wang T, Bian P, Wu Y, Wu L, Yu Z. The induction of bystander mutagenic effects in vivo by alpha-particle irradiation in whole Arabidopsis thaliana plants. Radiat Res 2010; 174:228-37; PMID:20681789; http://dx.doi.org/10.1667/RR2052.1
- Migicovsky Z, Yao Y, Kovalchuk I. Transgenerational phenotypic and epigenetic changes in response to heat stress in Arabidopsis thaliana. Plant Signal Behav 2014; 9:e27971; PMID:24513700; http://dx.doi.org/10.4161/psb.27971
- Rahavi SM, Kovalchuk I. Changes in homologous recombination frequency in Arabidopsis thaliana plants exposed to stress depend on time of exposure during development and on duration of stress exposure. Physiol Mol Biol Plant 2013; 19:479-88; PMID:24431516; http://dx.doi.org/10.1007/s12298-013-0197-z
- Yao Y, Kathiria P, Kovalchuk I. A systemic increase in the recombination frequency upon local infection of Arabidopsis thaliana plants with oilseed rape mosaic virus depends on plant age, the initial inoculum concentration and the time for virus replication. Front Plant Sci 2013; 4:61; PMID:23519399; http://dx.doi.org/10.3389/fpls.2013.00061
- Vilenchik MM, Knudson AG. Endogenous DNA double-strand breaks: production, fidelity of repair, and induction of cancer. Proc Natl Acad Sci U S A 2003; 100:12871-6; PMID:14566050; http://dx.doi.org/10.1073/pnas.2135498100
- Abe K, Osakabe K, Nakayama S, Endo M, Tagiri A, Todoriki S, Ichikawa H, Toki S. Arabidopsis RAD51C gene is important for homologous recombination in meiosis and mitosis. Plant Physiol 2005; 139:896-908; PMID:16169964; http://dx.doi.org/10.1104/pp.105.065243
- Stroud H, Greenberg MV, Feng S, Bernatavichute YV, Jacobsen SE. Comprehensive analysis of silencing mutants reveals complex regulation of the Arabidopsis methylome. Cell 2013; 152:352-64; PMID:23313553; http://dx.doi.org/10.1016/j.cell.2012.10.054
- Daxinger L, Kanno T, Bucher E, van der Winden J, Naumann U, Matzke AJ, Matzke M. A stepwise pathway for biogenesis of 24-nt secondary siRNAs and spreading of DNA methylation. EMBO J 2009; 28:48-57; PMID:19078964; http://dx.doi.org/10.1038/emboj.2008.260
- Wu L, Mao L, Qi Y. Roles of dicer-like and argonaute proteins in TAS-derived small interfering RNA-triggered DNA methylation. Plant Physiol 2012; 160:990-9; PMID:22846193; http://dx.doi.org/10.1104/pp.112.200279
- Yang DL, Zhang G, Tang K, Li J, Yang L, Huang H, Zhu JK. Dicer-independent RNA-directed DNA methylation in Arabidopsis. Cell Res 2016; 26:66-82; PMID:26642813; http://dx.doi.org/10.1038/cr.2015.145
- Kaina B. Mechanisms and consequences of methylating agent-induced SCEs and chromosomal aberrations: a long road traveled and still a far way to go. Cytogenet Genome Res 2004; 104:77-86; PMID:15162018; http://dx.doi.org/10.1159/000077469
- Tricker PJ, Gibbings JG, Rodriguez Lopez CM, Hadley P, Wilkinson MJ. Low relative humidity triggers RNA-directed de novo DNA methylation and suppression of genes controlling stomatal development. J Exp Botany 2012; 63:3799-813; PMID:22442411; http://dx.doi.org/10.1093/jxb/ers076
- Wei L, Gu L, Song X, Cui X, Lu Z, Zhou M, Wang L, Hu F, Zhai J, Meyers BC, et al. Dicer-like 3 produces transposable element-associated 24-nt siRNAs that control agricultural traits in rice. Proc Natl Acad Sci U S A 2014; 111:3877-82; PMID:24554078; http://dx.doi.org/10.1073/pnas.1318131111
- Song X, Li P, Zhai J, Zhou M, Ma L, Liu B, Jeong DH, Nakano M, Cao S, Liu C, et al. Roles of DCL4 and DCL3b in rice phased small RNA biogenesis. Plant J 2012; 69:462-74; PMID:21973320; http://dx.doi.org/10.1111/j.1365-313X.2011.04805.x
- Dunoyer P, Himber C, Voinnet O. DICER-LIKE 4 is required for RNA interference and produces the 21-nucleotide small interfering RNA component of the plant cell-to-cell silencing signal. Nat Genet 2005; 37:1356-60; PMID:16273107; http://dx.doi.org/10.1038/ng1675
- Vermeersch L, De Winne N, Nolf J, Bleys A, Kovarik A, Depicker A. Transitive RNA silencing signals induce cytosine methylation of a transgenic but not an endogenous target. Plant J 2013; 74:867-79; PMID:23480471; http://dx.doi.org/10.1111/tpj.12172
