ABSTRACT
Acetylcholinesterase (AChE), an acetylcholine-hydrolyzing enzyme, exists widely in plants, although its role in plant signal transduction is still unclear. We have hypothesized that the plant AChE regulates asymmetric distribution of hormones and substrates due to gravity stimulus, based on indirect pharmacological experiments using an AChE inhibitor. As a direct evidence for this hypothesis, our recent study has shown that AChE overexpression causes an enhanced gravitropic response in rice seedlings and suggested that the function of the rice AChE relates to the promotion of shoot gravitropism in the seedlings. Here, we report that AChE suppression inhibited shoot gravitropism in rice seedlings, as supportive evidence demonstrating the role of AChE as a positive regulator of shoot gravitropic response in plants.
Abbreviations
| ACh | = | Acetylcholine |
| ASCh | = | Acetylthiocholine |
| AChE | = | Acetylcholinesterase |
| DTNB | = | 5,5´-dithiobis-2-nitrobenzoic acid |
| UBQ | = | Ubiquitin |
Acetylcholine (ACh) is well-known as the neurotransmitter in the mammalian central and peripheral cholinergic nervous systems.Citation1 ACh has been also identified in a number of non-neuronal animal tissues and in fungi, bacteria, and plants.Citation2,3 ACh may play a universal role as an elementary molecular agent of irritability in animal, plant and microbial cells.Citation4 Although plants lack a nervous system, both ACh and AChE have been widely recognized in plants.Citation5 The function of the plant ACh and AChE is still unclear, despite more than 30 years of research. To understand those functions, we have been studying about physiological roles of plant AChE under gravitropic stimulus. Indeed plants under gravitropic stimulus showed asymmetric distribution of hormones and AChE activity.Citation6,7 When a plant is moved from the vertical orientation to a horizontal position, the stem shows curvature as it returns to a vertical position by response acting against gravistimulus. On the other hands, maize seedlings that were treated first with neostigmine bromide (AChE inhibitor) did not respond to the gravistimulus.Citation7 Furthermore, after the stimulus, the AChE activity was distributed asymmetrically at the interface between the stele and the cortex of the gravistimulated maize seedlings.Citation6 The gravitropic bending occurs because of an asymmetric distribution of the growth hormone auxin (indole-3-acetic acid, IAA).Citation8 We previously observed that gravistimulation leads to an asymmetric distribution of both IAA-inositol synthase and the AChE activities in maize and rice shoots.Citation9,10 In addition, its asymmetric distribution of both enzyme activities in maize and rice shoots was also inhibited by the treatment with neostigmine bromide.Citation9,10 The neostigmine bromide is an AChE inhibitor having no inhibitory effect on IAA metabolism in plants.Citation11 These findings suggest that AChE would play an important role in the gravitropic response of plants. To directly confirm our previous reports, the functionality of plant AChE under gravitropic stimulus was recently proved by overexpressing AChE gene in rice plants. AChE up-regulated plants showed an enhanced gravitropic response in rice seedlings.Citation12 In this report, in order to support our recent data, we silenced the AChE expression by RNAi in rice plants and assessed its impact on phenotypic characteristics under gravistimulation.
Generation and characterization of transgenic rice plants silencing rice AChE gene
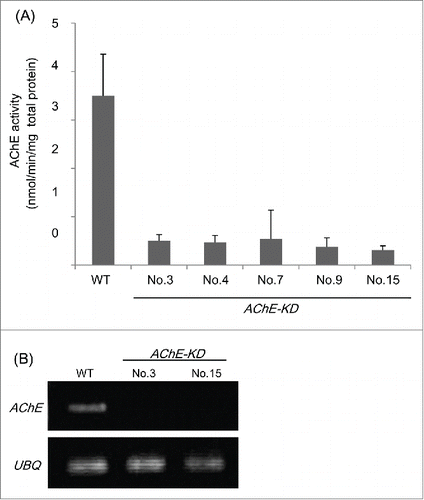
We generated transgenic rice plants in which the expression of the AChE gene (accession no. AK073754) is suppressed by RNAi techniques. The RNAi plasmid was constructed and designated AChE-KD. To generate inverted repeat regions from the gene-specific regions of AChE, 5′- and 3′-gene specific regions of rice AChE were connected. RNA-silencing construct carrying the inverted repeat of rice AChE fragments was made using the pANDA vector.Citation13,14 Transgenic rice plants were generated by Agrobacterium-mediated transformation of rice (cv. Nipponbare) calli as described in previous study.Citation12 Regenerated transgenic rice plants were grown in a large growth cabinet (Koitotron type KG: Koito Ind. Ltd. Co., Tokyo, Japan) at 28oC under 16 h light/d. Five independent transgenic lines were selected by AChE activity assay () and 2 T2 lines (AChE-KD No.3 and No.15) were chosen for RT-PCR. AChE-KD No.3 and No.15 silencing AChE gene showed an 85.5 and 90% decrease in levels of AChE activities respectively, compared with wild-type plants (). RNAi expression also caused reduced levels of gene expression compared with wild-type plants ().
Figure 1. Generation and characterization of transgenic rice plants silencing the rice AChE gene. RNAi-silenced line: AChE-KD. WT: wild type. (A) AChE activity in 4-d-old transgenic seedlings (T2 generation) measured by the DTNB method with acetylthiocholine (ASCh) as substrate according to our previous report.Citation12 Each textured bar represents the average of 3 replicates. Thin vertical bars represent standard errors. (B) RT-PCR analysis of mRNA expression. Designation to the left of each panel indicates primers used; UBQ indicates primers for the ubiquitin-encoding gene used as an internal control according to our previous report.Citation12
Shoot gravitropism in AChE- silencing plants
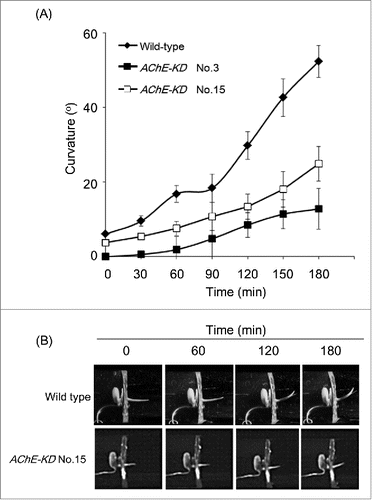
We placed transgenic seeds of AChE-KD No.3 and No.15 (T2 generation) in a tissue culture tube containing Murashige-Skoog nutrient medium and incubated in the dark at 28˚C for 4 d. For gravistimulation, the tube was oriented horizontally and curvature angles were measured on digital images taken 0, 30, 60, 90, 120, 150 and 180 min later as described in a previous study.Citation12 The shoot gravitropic response, relative to wild type, was inhibited when expression of the AChE gene was suppressed (). After 180 min of gravistimulation, wild-type seedlings bent upward at approximately 52.4°, whereas suppression of AChE caused greatly reduced gravitropic responses; the curvature of gene-silencing plantlets was only approximately 12.8° in AChE-KD No.3 and approximately 24.9° in AChE-KD No.15 after 180 min gravistimulation. The higher concentration of ACh has already been shown to possess an inhibitory effect on cell elongation.Citation15 In addition, Di Sansebastiano and colleagues recently reported that an ACh concentration beyond 50 mM appeared to negatively interfere with the auxin driven elongation process.Citation16 It is conceivable, therefore, that an ACh concertation in AChE- silencing plants might be higher than that in wild-type plants and its higher level of ACh might negatively regulate shoot gravitropism in AChE- silencing plants. In a previous report, we have already showed AChE overexpression causes an enhanced gravitropic response.Citation12 Taken together, these results strongly suggest that the rice AChE plays an important role in the gravitropic response and is a positive regulator of shoot gravitropism in the seedlings.
Figure 2. Shoot gravitropic responses of transgenic rice plants suppressing the expression of rice AChE gene. RNAi-silenced lines: AChE-KD No.3 and No.15. WT: wild type. (A) Quantification of shoot gravitropic responses in wild-type and transgenic rice plantlets silencing the rice AChE gene (T2 generation). Each data point represents measurement of 10 plantlets. Thin vertical bars represent standard errors. (B) Typical phenotypes of wild-type and transgenic rice plantlets silencing the rice AChE gene (T2 generation) under gravistimulation. Digital images were taken at designated time points to the upper of each panel.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. K. Shimamoto and Dr. D. Miki (Nara Institute of Science and Technology, Japan) for the kind gift of a pANDA vector. This research was supported by “Ground-based Research Program for Space Utilization (2006-2008)” (ID 77) promoted by Japan Space Forum.
References
- Beckmann J, Lips KS. The non-neuronal cholinergic system in health and disease. Pharmacology 2013; 92:286-302; PMID:24296914; http://dx.doi.org/10.1159/000355835
- Horiuchi Y, Kimura R, Kato N, Fujii T, Seki M, Endo T, Kato T, Kawashima K. Evolutional study on acetylcholine expression. Life Sci 2003; 72:1745-56; PMID:12559395; http://dx.doi.org/10.1016/S0024-3205(02)02478-5
- Yamada T, Fujii T, Kanai T, Amo T, Imanaka T, Nishimasu H, Wakagi T, Shoun H, Kamekura M, Kamagata Y, Kato T, Kawashima K. Expression of acetylcholine (ACh) and ACh-synthesizing activity in Archaea. Life Sci 2005; 77:1935-44; PMID:15936779; http://doi.org/10.1016/j.lfs.2005.01.026
- Roshchina VV. New trends and perspectives in the evolution of neurotransmitters in microbial, plant, and animal cells. Adv Exp Med Biol 2016; 874:25-77; PMID:26589213; http://link.springer.com/chapter/10.1007/978-3-319-20215-0_2
- Sagane Y, Nakagawa T, Yamamoto K, Michikawa S, Oguri S, Momonoki YS. Molecular characterization of maize acetylcholinesterase. A novel enzyme family in the plant kingdom. Plant Physiol 2005; 138:1359-71; PMID:15980188; http://dx.doi.org/10.1104/pp.105.062927
- Momonoki YS. Occurrence of acetylcholine-hydrolyzing activity at the stele-cortex interface. Plant Physiol 1992; 99:130-3; PMID:16668839
- Momonoki YS. Asymmetric distribution of acetylcholinesterase in gravistimulated maize seedlings. Plant Physiol 1997; 114:47-53; PMID:11536808; http://dx.doi.org/10.1104/pp.114.1.47
- Ross JJ, Wolbang CM. Auxin, gibberellins and the gravitropic response of grass leaf sheath pulvini. Plant Signal Behav 2008; 3:74-5; PMID:19704718; http://dx.doi.org/10.4161/psb.3.1.4929
- Momonoki YS, Hineno C, Noguchi K. Acetylcholine as a signaling system to environmental stimuli in plants. III. Asymmetric solute distribution controlled by ACh in gravistimulated maize seedlings. Plant Prod Sci 1998; 1:83-8; PMID:12162322; http://doi.org/10.1626/pps.1.83
- Momonoki YS, Kawai N, Takamure I, Kowalczyk S. Gravitropic response of acetylcholinesterase and IAA-inositol synthase in lazy rice. Plant Prod Sci 2000; 3:17-23; http://dx.doi.org/10.1626/pps.3.17
- Ballal S, Ellias R, Fluck R, Jameton R, Leber P, Lirio R, Salama D. The synthesis and bioassay of indole-3-acetylcholine. Plant Physiol Biochem 1993; 31:249-55.
- Yamamoto K, Shida S, Honda Y, Shono M, Miyake H, Oguri S, Sakamoto H, Momonoki YS. Overexpression of acetylcholinesterase gene in rice results in enhancement of shoot gravitropism. Biochem Biophys Res Commun 2015; 465:488-93; PMID:26277389; http://dx.doi.org/10.1016/j.bbrc.2015.08.044
- Miki D, Shimamoto K. Simple RNAi vectors for stable and transient suppression of gene function in rice. Plant Cell Physiol 2004; 45:490-5; PMID:15111724; http://dx.doi.org/10.1093/pcp/pch048
- Miki D, Itoh R, Shimamoto K. RNA silencing of single and multiple members in a gene family of rice. Plant Physiol 2005; 138:1903-13; PMID:16172097; http://doi.org/10.1104/pp.105.063933
- Lawson VR, Brady RM, Campbell A, Knox BG, Knox GD, Walls RL. Interaction of acetylcholine chloride with IAA, GA3 and red light in the growth of excised apical coleoptile segments. B Torrey Bot Club 1978; 105:187-191; http://www.jstor.org/stable/2484113
- Di Sansebastiano GP, Fornaciari S, Barozzi F, Piro G, Arru L. New insights on plant cell elongation: a role for acetylcholine. Int J Mol Sci 2014; 15:4565-82; PMID:24642879; http://dx.doi.org/10.3390/ijms15034565
