ABSTRACT
Plant NADPH oxidases are the major source of reactive oxygen species (ROS) that plays key roles as both signal and stressor in several plant processes, including defense responses against pathogens. ROS accumulation in root cells during arbuscular mycorrhiza (AM) development has raised the interest in understanding how ROS-mediated defense programs are modulated during the establishment of this mutualistic interaction. We have recently analyzed the expression pattern of 5 NADPH oxidase (also called RBOH) encoding genes in Medicago truncatula, showing that only one of them (MtRbohE) is specifically upregulated in arbuscule-containing cells. In line with this result, RNAi silencing of MtRbohE generated a strong alteration in root colonization, with a significant reduction in the number of arbusculated cells. On this basis, we propose that MtRBOHE-mediated ROS production plays a crucial role in the intracellular accommodation of arbuscules.
Roots of ∼80% of plant species in natural and agricultural systems are colonized by arbuscular mycorrhizal (AM) fungi, a crucial component of the plant microbiota.Citation1 In this mutualistic symbiosis the fungus delivers to the plant mineral nutrients, mainly phosphorus and nitrogen, in exchange for carbon.Citation2 Besides promoting plant growth, AM fungi sustain other important functions such as soil aggregation and water retention, tolerance to biotic and abiotic stresses and increase in plant biodiversity.Citation3 The clear ecological and economic importance of this symbiosis has strongly boosted the interest of the scientific community.
This very ancient and intimate plant-fungus association is thought to rely on a rigorous colonization program that leads the plant cell to accommodate intracellular fungal structures, including hyphae and highly branched arbuscules.Citation4 Root colonization is associated with massive rewiring of nutrient fluxes that guarantee reciprocal benefits to both the host plant and the fungus.Citation5 Important advances have been achieved in the last years on the molecular mechanisms governing the symbiosis; however, the way this mutualistic interaction overtakes plant defense remains largely obscure. In this frame, a key role is emerging for fungal effector proteins as communication factors, in analogy to several plant pathogenic interactions.Citation6,7 The SP7 secreted protein from Rhizophagus irregularis was indeed shown to interfere with the expression of plant defense genes.Citation6
On the plants side, a conserved defense response to pathogens is the production of Reactive Oxygen Species (ROS) which play a pivotal role in regulating numerous responses to biotic and abiotic stresses in plants. The complexity in ROS responses to diverse stimuli has been proposed to rely on the multiple regulatory mechanisms of ROS production via NADPH oxidases, one of the primary sources of ROS.Citation8 NADPH oxidases, known in plants as RBOH (respiratory burst oxidase homolog) catalyze the production of superoxide by transferring electrons from NADPH to molecular oxygen, with secondary generation of H2O2. They are encoded by a multigene family, with up to 10 different members in the model plant Arabidopsis thaliana.Citation9,10 Complex mechanisms of RBOH regulation, from transcriptional to post-translational level, occur and contribute to RBOH expression and function in an array of tissue types and developmental stages under various environmental conditions. Citation8,11
The activation of specific RBOH isoforms is responsible for ROS accumulation in several plant-pathogen interactionsCitation12,13 and in the symbiotic interaction between legumes and nitrogen-fixing rhizobia.Citation14,15,16
H2O2 has been detected in root cells colonized by AM fungi.Citation17,18,19 Interestingly, the up-regulation of fungal genes implicated in oxidative stress defense has also been reported in mature mycorrhizasCitation19,20 suggesting that protection against localized ROS-based host defense responses may be involved in arbuscule formation and/or maintenance. Starting from the hypothesis that plant RBOH could be good candidates for H2O2 production in arbuscular mycorrhizas, we have analyzed in a recent publication the spatio-temporal expression profiles of 5 Rboh genes from the model legume Medicago truncatula (MtRbohA, MtRbohB, MtRbohE, MtRbohG, MtRbohF) during the establishment of the AM symbiosis.Citation21 MtRboh transcript levels did not drastically change in total RNA extractions from whole mycorrhizal and non mycorrhizal roots in a time course experiment of root colonization (7, 14, 28 and 60 d post-inoculation), with the highest expression level always observed for MtRbohG. This is not surprising, because AM colonization is an asyncronous process and plant responses often develop on a small local scale in mycorrhizal roots. To achieve a more detailed view of gene expression pattern we used a complementary cellular and molecular approach that allowed transcript localization in different cell types. The analysis of Agrobacterium rhizogenes-transformed roots expressing a GUS transcriptional fusion construct with MtRboh promoters showed that all genes are expressed in the central cylinder (in both mycorrhizal and non mycorrhizal conditions), underlying the importance of RBOH and ROS in cell wall metabolism.Citation22 This approach also highlighted the expression of MtRbohE in cells containing arbuscules.
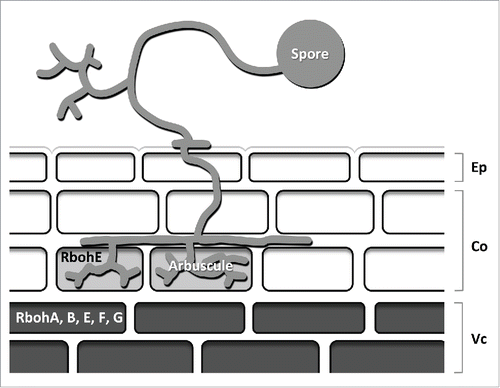
The laser microdissection technique clearly showed the expression of 2 genes, MtRbohG and MtRbohE, in cortical cells, whether or not they were colonized by fungal hyphae. Thus, this technique turned out to be more sensitive than the GUS histochemical assay since the MtRbohG promoter activity was never observed in cortical cells. Remarkably, MtRbohE transcripts appeared more abundant in arbuscule-containing cells compared to adjacent non colonized cells, supporting the results obtained with the GUS assay. A summary of the expression pattern of the 5 analyzed MtRboh genes in a mycorrhizal root is shown in .
Figure 1. Scheme of the expression profiles of the investigated MtRboh genes in a mycorrhizal root. Based on histochemical GUS assays MtRbohA, B, E, F and G are expressed in the vascular cylinder while MtRbohE is also expressed in arbuscule-containing cells. Ep: epidermis; Co: cortex; Vc: vascular cylinder.
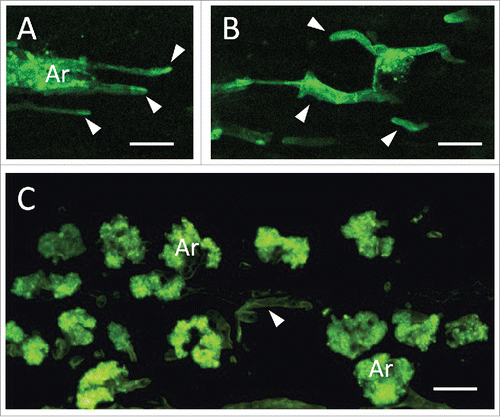
To further clarify the role of MtRbohE, we generated RNAi lines. While RbohE-silenced plants showed a normal nodulation phenotype, an altered AM colonization pattern was observed in the root cortex, with fewer arbuscules and more abundant intercellular hyphae, compared to control roots (). Altogether our data indicate the transient upregulation of MtRbohE expression in arbusculated cells and suggest a role for MtRbohE in arbuscule accommodation within cortical cells.
Figure 2. Altered arbuscular mycorrhizal colonization in MtRbohE RNAi-silenced lines of Medicago truncatula. MtRbohE-silenced plants generated an abnormal colonization pattern (A, B), with fewer arbuscules (Ar) and more frequent intercellular hyphae (arrowheads), compared to wild type roots (C). Confocal images of WGA-FITC stained fungal structures in root longitudinal sections. Bars = 20µm.
Our results integrate those from Arthikala and colleagues who recently characterized RBOH in arbuscular mycorrhizas of another legume plant: the PvRbohB gene from Phaseolus vulgaris (homolog of MtRbohG) turned out to act as a negative regulator of the AM symbiosis while it is required for root infection by rhizobia.Citation23,24
Although only 2 Rboh genes from 2 different legumes have been characterized so far Citation10,14,23,24 these findings suggest that different gene members of the RBOH family play distinct functions in the AM symbiosis; moreover, some of them may even have opposite functions (promotion versus inhibition) in the 2 types of root symbioses. Interestingly, in the case of MtRbohE silencing, no phenotype has been observed during rhizobial symbiosis.Citation21 The temporal and spatial fine tuning of RBOH-derived ROS, seems therefore to contribute to the establishment of fully functional interactions in these plant-microbe associations. The AM symbiont may also participate to ROS production by specific fungal NADPH oxidases (also known as Nox). Nox, which also belong to a gene family with up to 3 (A, B and C) classes, play a key role in fungal cellular differentiation and development.Citation25 Interestingly, a NoxA gene was shown to be critical for maintaining a mutualistic symbiosis between the fungal endophyte Epichloë festucae and its host plant Lolium perenne.Citation26,27 Molecular and in silico analyses revealed that the AM fungus Rhizophagus irregularis possess Nox genes, belonging to class A and B, which are expressed in arbuscule-containing cells (Fiorilli and Lanfranco, unpublished results). A fungal contribution to NADPH-oxidases-related processes in the in planta phase can thus be envisaged.
Future challenges will be to decipher how the NADPH-oxidases activities not only from the plant but also from the fungal partner exert their control over the AM colonization process eventually interacting with other signals.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Research was funded by the BIOBIT-Converging Technology project (WP2) to LL, the University grant (60%) to LL and AG, the BLAN07-2_182872 ANR research program to AP.
References
- Lanfranco L, Young P. Genetic and genomic glimpses of the elusive arbuscular mycorrhizal fungi. Curr Opin Plant Biol 2012; 15:454-61; PMID:22673109; http://dx.doi.org/10.1016/j.pbi.2012.04.003
- van der Heijden MGA, Martin F, Selosse MA, Sanders IR. Mycorrhizal ecology and evolution: The past, the present and the future. New Phytol 2015; 205:1406-23; PMID:25639293; http://dx.doi.org/10.1111/nph.13288
- Gianinazzi S, Gollotte A, Binet MN, van Tuinen D, Redecker D, Wipf D. Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 2010; 20:519-30; PMID:20697748; http://dx.doi.org/10.1007/s00572-010-0333-3
- Genre A, Russo G. Does a common pathway transduce symbiotic signals in plant-microbe interactions? Front Plant Sci 2016; 7:96; PMID:26909085; http://dx.doi.org/10.3389/fpls.2016.00096
- Walder F, van der Heijden M. Regulation of resource exchange in the arbuscular mycorrhizal symbiosis. Nature Plants 2015; 1:15159
- Kloppholz S, Kuhn H, Requena N. A secreted fungal effector of Glomus intraradices promotes symbiotic biotroph. Curr Biol 2011; 21:1-6; PMID:21129968; http://dx.doi.org/10.1016/j.cub.2011.06.044
- Sedzielewska-Toro K, Delaux PM. Mycorrhizal symbioses: today and tomorrow. New Phytol 2016; 209:917-20; PMID:26756534; http://dx.doi.org/10.1111/nph.13820
- Baxter A, Mittler R, Suzuki N. ROS as key players in plant stress signalling. J Exp Bot 2014; 65:1229-40; PMID:24253197; http://dx.doi.org/10.1093/jxb/ert375
- Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R. Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol 2011; 14:691-9; PMID:21862390; http://dx.doi.org/10.1016/j.pbi.2011.07.014
- Marino D, Dunand C, Puppo A, Pauly N. A burst of plant NADPH oxidases. Trends Plant Sci 2012; 17:9-15; PMID:22037416; http://dx.doi.org/10.1016/j.tplants.2011.10.001
- Scheler C, Durner J, Astier J. Nitric oxide and reactive oxygen species in plant biotic interactions. Curr Opin Plant Biol 2013; 16:534-9; PMID:23880111; http://dx.doi.org/10.1016/j.pbi.2013.06.020
- Torres MA. ROS in biotic interactions. Physiol Plant 2010; 138:414-29; PMID:20002601; http://dx.doi.org/10.1111/j.1399-3054.2009.01326.x
- Lehmann S, Mario Serrano M, L'Haridon F, Tjamos SE, Metraux J-P. Reactive oxygen species and plant resistance to fungal pathogens. Phytochemistry 2015; 112:54-62; PMID:25264341; http://dx.doi.org/10.1016/j.phytochem.2014.08.027
- Montiel J, Nava N, Cárdenas L, Sánchez-López R, Arthikala MK, Santana O, Sanchez F, Quinto C. 2012. A Phaseolus vulgaris NADPH oxidase gene is required for root infection by Rhizobia. Plant Cell Physiol 2012; 53:1751-67; PMID:22942250; http://dx.doi.org/10.1093/pcp/pcs120
- Marino D, Andrio E, Danchin EGJ, Oger E, Gucciardo S, Lambert A, Puppo A, Pauly N. A Medicago truncatula NADPH oxidase is involved in symbiotic nodule functioning. New Phytol 2011; 189:580-92; PMID:21155825; http://dx.doi.org/10.1111/j.1469-8137.2010.03509.x
- Puppo A, Pauly N, Boscari A, Manodn K, Brouquisse R. Hydrogen peroxide and nitric oxide: key regulators of the legume-Rhizobium and mycorrhizal symbiosis. Antioxid Redox Sign 2013; 18:1-18; PMID:23249379; http://dx.doi.org/10.1089/ars.2012.4980
- Salzer P, Corbiere H, Boller T. Hydrogen peroxide accumulation in Medicago truncatula roots colonized by the arbuscular mycorrhiza-forming fungus Glomus intraradices. Planta 1999; 208:319-25; http://dx.doi.org/10.1007/s004250050565
- Fester T, Hause G. Accumulation of reactive oxygen species in arbuscular mycorrhizal roots. Mycorrhiza 2005; 15:373-9; PMID:15875223; http://dx.doi.org/10.1007/s00572-005-0363-4
- Lanfranco L, Novero M, and Bonfante P. The mycorrhizal fungus Gigaspora margarita possesses a CuZn superoxide dismutase that is up-regulated during symbiosis with legume hosts. Plant Physiol 2005; 137:1319-30; PMID:15749992; http://dx.doi.org/10.1104/pp.104.050435
- Benabdellah K, Azcon-Aguilar C, Valderas A, Speziga D, Fitzpatrick TB, and Ferrol N. GintPDX1 encodes a protein involved in vitamin B6 biosynthesis that is up-regulated by oxidative stress in the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol 2009; 184:682-93; PMID:19674326; http://dx.doi.org/10.1111/j.1469-8137.2009.02978.x
- Belmondo S, Calcagno C, Genre A, Puppo A, Pauly N, Luisa Lanfranco L. The Medicago truncatula MtRbohE gene is activated in arbusculated cells and is involved in root cortex colonization. Planta 2016; 243:251-62; PMID:26403286; http://dx.doi.org/10.1007/s00425-015-2407-0
- Kärkönen A, Kuchitsu K. Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry 2015; 112:22-32; PMID:Can't; http://dx.doi.org/10.1016/j.phytochem.2014.09.016
- Arthikala M-K, Montiel J, Nava N, Santana O, Sánchez-López R, Cárdenas L, Quinto C. PvRbohB negatively regulates Rhizophagus irregularis colonization in Phaseolus vulgaris. Plant Cell Physiol 2013; 54:1391-402; PMID:23788647; http://dx.doi.org/10.1093/pcp/pct089
- Arthikala M-K, Sánchez-López R, Nava N, Santana O, Cárdenas L, Quinto C. RbohB, a Phaseolus vulgaris NADPH oxidase gene, enhances symbiosome number, bacteroid size, and nitrogen fixation in nodules and impairs mycorrhizal colonization. New Phytol 2014; 202:886-900; PMID:24571730; http://dx.doi.org/10.1111/nph.12714
- Tudzynski P, Heller J, Siegmund U. Reactive oxygen species generation in fungal development and pathogenesis. Curr Opin Microbiol 2012; 15:653-9; PMID:23123514; http://dx.doi.org/10.1016/j.mib.2012.10.002
- Tanaka A, Christensen MJ, Takemoto D, Park P, Scott B. Reactive oxygen species play a role in regulating a fungus-perennial ryegrass mutualistic interaction. Plant Cell 2006; 18:1052-66; PMID:16517760; http://dx.doi.org/10.1105/tpc.105.039263
- Tanaka A, Takemoto D, Hyon GS, Park P, and Scott B. NoxA activation by the small GTPase RacA is required to maintain a mutualistic symbiotic association between Epichloe festucae and perennial ryegrass. Mol Microbiol 2008; 68:1165-78; PMID:18399936; http://dx.doi.org/10.1111/j.1365-2958.2008.06217.x
