ABSTRACT
WRKY proteins, which belong to a large family of plant-specific transcription factors, play important roles in plant defenses against pathogens and herbivores by regulating defense-related signaling pathways. Recently, a rice WRKY transcription factor OsWRKY53 has been reported to function as a negative feedback modulator of OsMPK3/OsMPK6 and thereby to control the size of the investment a rice plant makes to defend against a chewing herbivore, the striped stem borer Chilo suppressalis. We investigated the performance of a piecing-sucking herbivore, the brown planthopper (BPH) Nilaparvata lugens, on transgenic plants that silence or overexpress OsWRKY53, and found that OsWRKY53 activates rice defenses against BPH by activating an H2O2 burst and suppressing ethylene biosynthesis. These findings suggest that OsWRKY53 functions not only as a regulator of plants' investment in specific defenses, but also as a switch to initiate new defenses against other stresses, highlighting the versatility and importance of OsWRKY53 in herbivore-induced plant defenses.
Abbreviations
| SSB | = | striped stem borer |
| BPH | = | brown planthopper |
| MPK | = | mitogen-activated protein kinase |
| JA | = | jasmonic acid |
| JA-Ile | = | jasmonoyl-isoleucine |
| SA | = | salicylic acid |
| WT | = | wild type |
| Glc | = | glucose |
| GOX | = | glucose oxidase |
| Buf | = | buffer |
| FW | = | fresh weight |
| LSM | = | least squares means |
| FDR | = | false discovery rate |
Plants have evolved well-developed defensive systems to cope with herbivore challenges. They can recognize specific herbivory stimuli and respond effectively by activating a defense-related signaling network, which consists mainly of mitogen-activated protein kinase (MPK) cascades and jasmonic acid (JA)-, jasmonoyl-isoleucine (JA-Ile)-, salicylic acid (SA)-, ethylene- and H2O2-mediated signaling pathways.Citation1,2 The activated signaling network will lead to the expression of numerous defense-related genes and the accumulation of defense compounds.Citation1,2 During this process, transcription factors play a central role.Citation3 WRKY proteins, which belong to a large family of plant-specific transcription factors, can bind W-box cis-elements (TTGAC(C/T)) in the promoters of their target genes and regulate the expression of these genes by acting as activators or repressors.Citation4,5 WRKYs have been reported to play an important role in plant defenses against pathogens and herbivores by regulating the defense-related signaling network; they function both up- and down-stream of components of the network, such as receptors, protein kinases and phytohormones.Citation5 Recently, we found that OsWRKY53, which was slowly induced by infestation of a chewing herbivore, the striped stem borer (SSB) Chilo suppressalis, negatively modulates OsMPK3/OsMPK6, thereby reducing JA, JA-Ile and ethylene induction as well as the resistance of rice to SSB.Citation6 This negative feedback regulation of OsWRKY53 on OsMPK3/OsMPK6 was regarded as a strategy by which plants control their defensive investment.Citation6 However, whether and how the OsWRKY53-mediated defense strategy influences the performance of other rice insect pests remain unknown.
The brown planthopper (BPH) Nilaparvata lugens is one of the most important insect pests on rice. It feeds on the plant's phloem sap, and because it decreases leaf area, plant height, dry weight, chlorophyll contents, and photosynthetic rate, it causes significant yield loss.Citation7,8 Previous studies have revealed that rice has different mechanisms that help it resist SSB and BPH.Citation9-11 Therefore, we investigated the effect of the OsWRKY53-mediated defense on the performance of BPH. In the study, the plant growth condition, plant age used for experiments and plant treatments were the same as described in Hu et al.Citation6 Like infestation by SSB larvae,Citation6 gravid female BPH adult infestation at a late stage (>8 h) also significantly up-regulated transcriptional levels of OsWRKY53 (). To analyze whether OsWRKY53 affects the ability of rice to resist BPH, the performance of BPH was assessed on wild type (WT) rice plants and transgenic lines that were either silenced in their OsWRKY53 (ir-wrky lines, ir-14 and ir-29) or overexpressed it (oe-WRKY lines, oe-5 and oe-6). When different rice genotypes were exposed to one BPH colony, BPH females preferred to feed and lay eggs on ir-wrky lines over WT plants (). Consistently, BPH females were less frequently observed and laid fewer eggs on oe-WRKY lines than on WT plants (). Similarly, BPH nymphs fed on ir-wrky lines showed higher survival rates than those fed on WT plants, whereas nymphs fed on oe-WRKY lines had significantly lower survival rates compared to those fed on WT plants (). Additionally, the survival rates of BPH eggs were remarkably reduced on oe-WRKY lines (). The tolerance of transgenic lines and WT plants to female BPH adults was also different. Eleven days after infestation by 15 gravid female BPH adults, ir-wrky plants had completely wilted, whereas WT plants only showed necrosis in the outer leaf sheaths; 18 d after infestation, the WT plants had died, whereas the oe-WRKY plants remained green and healthy (). These results indicate that OsWRKY53 positively regulates resistance in rice to BPH.
Figure 1. OsWRKY53 positively regulates the ability of rice to resist brown planthopper (BPH). (A) Mean transcript levels (+SE, n = 5) of OsWRKY53 in rice stems that were infested by 15 gravid female BPH adults. Non-infested plant stems were covered with an empty glass cage. Transcript levels were analyzed by quantitative RT-PCR. Asterisks represent significant differences between treatments and controls at the indicated times (2-way analysis of variance, followed by pairwise comparisons of least squares means (LSM), P values were corrected by the false discovery rate (FDR) method; *, P< 0.05). (B) Oviposition marks (a, indicated by arrows), eggs (b) and female adults (c) of BPH. (C) to (F) Mean number of female BPH adults per plant (+SE, n = 10) on pairs of plants (wild type (WT) versus ir-14, ir-29, oe-5 and oe-6, respectively), 1–48 h after pairs were exposed to insects. Inserts: mean percentage (+SE, n = 10) of BPH eggs per plant on pairs of plants as stated above, 48 h after the release of BPH. Asterisks indicate a significant preference within each combination and time point (Wald test, *, P < 0.05, **, P < 0.01). (G) and (H) Mean survival rate (+SE, n = 10) of BPH nymphs that fed on ir-wrky lines, oe-WRKY lines or WT plants 1-12 d after the start of feeding. Inserts: mean hatching rate (+SE, n = 6) of BPH eggs on ir-wrky lines, oe-WRKY lines or WT plants. Asterisks represent significant differences between transgenic lines and WT plants (generalized linear model [family: Binomial or Quasibinomial], followed by pairwise comparisons of LSM, P values were corrected by FDR method;*, P < 0.05, **, P < 0.01). (I) and (J) Damaged phenotypes of ir-wrky, oe-WRKY lines and WT plants that were individually infested by 15 BPH female adults for 11 (I) or 18 (J) days (n = 20).
![Figure 1. OsWRKY53 positively regulates the ability of rice to resist brown planthopper (BPH). (A) Mean transcript levels (+SE, n = 5) of OsWRKY53 in rice stems that were infested by 15 gravid female BPH adults. Non-infested plant stems were covered with an empty glass cage. Transcript levels were analyzed by quantitative RT-PCR. Asterisks represent significant differences between treatments and controls at the indicated times (2-way analysis of variance, followed by pairwise comparisons of least squares means (LSM), P values were corrected by the false discovery rate (FDR) method; *, P< 0.05). (B) Oviposition marks (a, indicated by arrows), eggs (b) and female adults (c) of BPH. (C) to (F) Mean number of female BPH adults per plant (+SE, n = 10) on pairs of plants (wild type (WT) versus ir-14, ir-29, oe-5 and oe-6, respectively), 1–48 h after pairs were exposed to insects. Inserts: mean percentage (+SE, n = 10) of BPH eggs per plant on pairs of plants as stated above, 48 h after the release of BPH. Asterisks indicate a significant preference within each combination and time point (Wald test, *, P < 0.05, **, P < 0.01). (G) and (H) Mean survival rate (+SE, n = 10) of BPH nymphs that fed on ir-wrky lines, oe-WRKY lines or WT plants 1-12 d after the start of feeding. Inserts: mean hatching rate (+SE, n = 6) of BPH eggs on ir-wrky lines, oe-WRKY lines or WT plants. Asterisks represent significant differences between transgenic lines and WT plants (generalized linear model [family: Binomial or Quasibinomial], followed by pairwise comparisons of LSM, P values were corrected by FDR method;*, P < 0.05, **, P < 0.01). (I) and (J) Damaged phenotypes of ir-wrky, oe-WRKY lines and WT plants that were individually infested by 15 BPH female adults for 11 (I) or 18 (J) days (n = 20).](/cms/asset/57d2e8a6-f048-4ccb-a170-64b8eb178a75/kpsb_a_1169357_f0001_oc.gif)
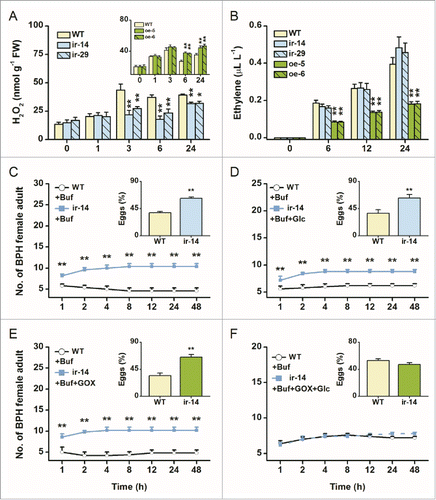
It has been reported that H2O2 and ethylene play a crucial role helping rice to resist BPH.Citation9,11,Citation12 Thus, we determined the basal and elicited levels of H2O2 and ethylene in WT plants and transgenic lines using the same methods as described in Lu et al.Citation11 Although basal H2O2 levels were similar in all lines, levels of H2O2 after BPH infestation were significantly decreased and increased in ir-wrky (ir-14, ir-29) and oe-WRKY (oe-5, oe-6) lines, respectively, compared to those in WT plants (). BPH-elicited ethylene levels in oe-WRKY lines were obviously lower than those in WT plants, but no difference was observed between ir-wrky lines and WT plants (). These differences suggest that OsWRKY53 positively regulated H2O2 production but negatively mediated ethylene biosynthesis. To determine if the improved performance of BPH on ir-wrky lines was caused by reduced H2O2 production, we conducted a complementation experiment with H2O2, and chose the BPH feeding and oviposition preference as a bioassay index. When the ir-14 line was treated with glucose oxidase (GOX) and glucose (Glc) solution, which can generate H2O2 in situ, the increase in the preference of BPH for ir-wrky lines was abolished: BPH female adults showed equal attraction to and oviposition preference for ir-wrky lines and WT plants without the supplemented H2O2 (); in contrast, the exogenous application of (GOX) or Glc solution alone on the transgenic plants did not alter the preference of BPH for WT and transgenic plants (). Previous studies have demonstrated that the ethylene pathway negatively regulates the resistance of rice to BPH.Citation12 Taken together, these results suggest that the decreased resistance of ir-wrky lines to BPH is largely owing to low H2O2 levels, whereas the improved resistance of oe-WRKY lines is probably because of high H2O2 and low ethylene levels, both of which were mediated by OsWRKY53.
Figure 2. Exogenous application of H2O2 complements resistance to rice brown planthopper (BPH) in ir-wrky lines. (A) and (B) Mean levels (+SE, n = 5) of H2O2 (A) and ethylene (B) in ir-wrky and oe-WRKY lines and in wild-type (WT) plants that were individually infested by 15 female BPH adults. FW, fresh weight. Asterisks indicate significant differences in ir-wrky and oe-WRKY lines compared with WT plants (2-way analysis of variance , followed by pairwise comparisons of least squares means, P values were corrected by the false discovery rate method; *, P < 0.05, **, P < 0.01). (C) to (F) Mean number of adult female BPH per plant (+SE, n = 10) on pairs of plants, WT plants treated with 400 μL of 20 mM sodium phosphate buffer (pH 6.5) vs. ir-14 plants treated with 400 μL of the buffer (C), ir-14 plants treated with 400 μL of Glc (25 mM) in the buffer (D), ir-14 plants treated with 400 μL of GOX (50 units mL-1) in the buffer (E), and ir-14 plants treated with 400 μL of GOX (50 units mL−1) and Glc solution (25 mM) in the buffer (F), respectively, 1–48 h after the release of female adults. Inserts: mean percentage (+SE, n = 10) of BPH eggs per plant on pairs of plants as stated above, 48 h after the release of BPH. Buf, buffer; Glc, glucose; GOX, glucose oxidase. Asterisks indicate a significant preference within each combination and time point (Wald test, *P < 0.05; **P < 0.01).
OsWRKY53 has been found to act as an early suppressor of SSB-induced defenses and thereby enables rice plants to control how much they allocate for defense.Citation6 Here, we demonstrated that OsWRKY53 activates plant defenses in response to BPH infestation and that OsWRKY53 increases the accumulation of H2O2 and decreases ethylene levels (). It has been well documented that piercing-sucking insects, including BPH, induced plant defense responses that were similar to those induced by pathogens;Citation13 in addition, OsWRKY53 is known to be induced by pathogen infection and to play an important role in helping rice resist pathogens by activating defense-related genes.Citation14,15 Moreover, plants in nature are often attacked simultaneously by multiple herbivore species with different feeding habits, and herbivore infestation usually causes plants to become more susceptible to pathogens. Therefore, in addition to regulating plants' investment for specific defenses, OsWRKY53 may also be recruited by plants infested by SSB at a relatively late stage to control possible pathogens (and piercing-sucking herbivores) by regulating pathogen defense-related signaling pathways, such as H2O2 and ethylene pathways.Citation16,17 This suggests that OsWRKY53 is a versatile switch that can turn on or off different signaling pathway-dependent defenses: “off” will activate the chewing herbivore-induced defense and suppress the defense against pathogens and piercing-sucking herbivores, whereas “on” will inhibit the former and activate the later. This strategy could cause plants to control their investment for specific defenses and concurrently move the energy to defend the coming stresses, thereby leading to plants high fitness.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The study was jointly sponsored by the Innovation Research Team Program of the National Natural Science Foundation of China (31321063), the National Natural Science Foundation of China (31330065), the Special Fund for Agro-scientific Research in the Public Interest of Zhejiang (2014C22004), and the China Agriculture Research System (CARS–01–21). We thank Maxime Hervé and Xi Zhang for their statistic assistance and Emily Wheeler for editorial assistance.
References
- Wu J, Baldwin IT. New insights into plant responses to the attack from insect herbivores. Annu Rev Genet 2010; 44:1-24; PMID:20649414; http://dx.doi.org/10.1146/annurev-genet-102209-163500
- Erb M, Meldau S, Howe GA. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci 2012; 17:250-9; PMID:22305233; http://dx.doi.org/10.1016/j.tplants.2012.01.003
- Woldemariam MG, Baldwin IT, Galis I. Transcriptional regulation of plant inducible defenses against herbivores: a mini-review. J Plant Interact 2011; 6:113-9; http://dx.doi.org/10.1080/17429145.2010.544779
- Rushton PJ, Somssich IE, Ringler P, Shen QJ. WRKY transcription factors. Trends Plant Sci 2010; 15:247-58; PMID:20304701; http://dx.doi.org/10.1016/j.tplants.2010.02.006
- Bakshi M, Oelmuller R. WRKY transcription factors: Jack of many trades in plants. Plant Signal Behav 2014; 9:e27700; PMID:24492469; http://dx.doi.org/10.4161/psb.27700
- Hu L, Ye M, Li R, Zhang T, Zhou G, Wang Q, Lu J, Lou Y. The rice transcription factor WRKY53 suppresses herbivore-induced defenses by acting as a negative feedback modulator of mitogen-activated protein kinase activity. Plant Physiol 2015; 169:2907-21; PMID:26453434; http://dx.doi.org/10.1104/pp.15.00646
- Watanabe T, Kitagawa H. Photosynthesis and translocation of assimilates in rice plants following phloem feeding by the planthopper Nilaparvata lugens (Homoptera : Delphacidae). J Econ Entomol 2000; 93:1192-8; PMID:10985030; http://dx.doi.org/10.1603/0022-0493-93.4.1192
- Rubia-Sanchez E, Suzuki Y, Miyamoto K, Watanabe T. The potential for compensation of the effects of the brown planthopper Nilaparvata lugens Stal (Homoptera : Delphacidae) feeding on rice. Crop Protect 1999; 18:39-45; http://dx.doi.org/10.1016/S0261-2194(98)00087-8
- Zhou G, Qi J, Ren N, Cheng J, Erb M, Mao B, Lou Y. Silencing OsHI-LOX makes rice more susceptible to chewing herbivores, but enhances resistance to a phloem feeder. Plant J 2009; 60:638-48; PMID:19656341; http://dx.doi.org/10.1111/j.1365-313X.2009.03988.x
- Qi J, Zhou G, Yang L, Erb M, Lu Y, Sun X, Cheng J, Lou Y. The chloroplast-localized phospholipases D alpha4 and alpha5 regulate herbivore-induced direct and indirect defenses in rice. Plant Physiol 2011; 157:1987-99; PMID:21984727; http://dx.doi.org/10.1104/pp.111.183749
- Lu J, Ju H, Zhou G, Zhu C, Erb M, Wang X, Wang P, Lou Y. An EAR-motif-containing ERF transcription factor affects herbivore-induced signaling, defense and resistance in rice. Plant J 2011; 68:583-96; PMID:21831212; http://dx.doi.org/10.1111/j.1365-313X.2011.04709.x
- Lu J, Li J, Ju H, Liu X, Erb M, Wang X, Lou Y. Contrasting effects of ethylene biosynthesis on induced plant resistance against a chewing and a piercing-sucking herbivore in rice. Mol Plant 2014; 7:1670-82; PMID:25064847; http://dx.doi.org/10.1093/mp/ssu085
- Kaloshian I, Walling LL. Hemipterans as plant pathogens. Annu Rev Phytopathol 2005; 43:491-521; PMID:16078893; http://dx.doi.org/10.1146/annurev.phyto.43.040204.135944
- Chujo T, Takai R, Akimoto-Tomiyama C, Ando S, Minami E, Nagamura Y, Kaku H, Shibuya N, Yasuda M, Nakashita H, et al. Involvement of the elicitor-induced gene OsWRKY53 in the expression of defense-related genes in rice. Biochim Biophys Acta 2007; 1769:497-505; PMID:17532485; http://dx.doi.org/10.1016/j.bbaexp.2007.04.006
- Chujo T, Miyamoto K, Ogawa S, Masuda Y, Shimizu T, Kishi-Kaboshi M, Takahashi A, Nishizawa Y, Minami E, Nojiri H, et al. Overexpression of phosphomimic mutated OsWRKY53 leads to enhanced blast resistance in rice. PLoS One 2014; 9:e98737; PMID:24892523; http://dx.doi.org/10.1371/journal.pone.0098737
- Vellosillo T, Vicente J, Kulasekaran S, Hamberg M, Castresana C. Emerging complexity in reactive oxygen species production and signaling during the response of plants to pathogens. Plant Physiol 2010; 154:444-8; PMID:20921160; http://dx.doi.org/10.1104/pp.110.161273
- Shen X, Liu H, Yuan B, Li X, Xu C, Wang S. OsEDR1 negatively regulates rice bacterial resistance via activation of ethylene biosynthesis. Plant Cell Environ 2011; 34:179-91; PMID:20807375; http://dx.doi.org/10.1111/j.1365-3040.2010.02219.x
