ABSTRACT
Macaranga bancana is considered as a successful pioneer plant species. Usually found in disturbed and open areas, most of the current research focused on its relations with ants. One of the unique feature of the plants is that the seedling leaves are red, resembling and almost matching the background. Using a portable spectrometer, we measured the color reflectance of M. bancana seedlings (less than 20 cm in height). We also measured the leaf litter reflectance, adult M. bancana leaves and also seedlings of several other species found in the vicinity of M. bancana seedlings. The reflectances of M. bancana seedlings are very similar to that of the leaf litter background. We suggest that this cryptic coloration is crucial during the early stages of the plant when it still cannot rely on the protection of ants.
Plants are sessile organisms that rely on multiple signaling systems. Plants rely on both physical and chemical signals either to attract pollinators and seed dispersers, or to defend from grazing herbivores. Plants also use cryptic coloration as camouflage from grazing herbivores.Citation1-3 There are also plants that use aposematic coloration as a warning signal.Citation4,5
There are a few species of plants that uses different color strategies throughout their ontogeny. For example, Pseudopanax crassifolius adapts cryptic colorations during the seedling stage and changes to aposematic colors during the juvenile stage.Citation4 Another unique example is the Elaeocarpus hookerianus, a heteroblastic plant that has varied leaf shapes and brown mottled colors mimicking the leaf litter.Citation1 La Rocca et al.Citation6 reported the dual functioned colors of Erythronium dens-canis. This amazing understory Lilly has complex leaf pattern to remain cryptic to herbivores, while certain portions of the reflective colors function as attractants for pollinators. Corydalis benecincta, an alpine plant with dimorphic colors (green and gray), in which the gray colored variety had fewer predation effect while still retaining a similar photosynthetic performance and visual cues to attract pollinators with the green colored variety.Citation3
These visual defenses (either to remain cryptic or aposematic) are plant and environment specific. Most of the recorded plants originate from the temperate climate and in some cases, are evolutionary linked with the environment or to a certain animal taxon. There is a scarcity of examples of such visual defenses from the tropics. They include being red/brown to look dead,Citation7 delayed greening,Citation8 red leaf undersides that defend from fungal attacks,Citation9 different adaxial and abaxial leaf colors that undermine herbivorous insect camouflageCitation10 and aposematism.Citation11
Macaranga bancana (Miq.) Müll.Arg. of the Euphorbiaceae is a common tree species in the South East Asia region, especially in Malaysia. Previously, the species was commonly mistaken as M. triloba, which has an almost similar appearance except for lacking hollow twigs. The seedlings are often described as reddish purple in color with prominent glands lining leaf margin.
Considered as a pioneer species, M. bancana are usually found along roads and recently disturbed areas.Citation12 The species is also well known as an example of myrmecophytic relation with ants. The hollow space within the twigs provides excellent shelter for ants and in return, the ants provide protection to the plants. Although there had been numerous studies on the myrmecophyte relation with ants,Citation13-15 there is almost no information on other types of adaptive strategies employed by M. bancana.
Here we suggest that M. bancana utilizes different adaptive tactics throughout its ontogeny. A shade intolerant species, with a fast sprouting and growth, M. bancana has been known to thrive and occupy disturbed habitats and open areas with access to greater sunlight. Using spectral analysis, we measured the color reflectance of adult and seedlings of M. bancana. We also measured the spectral reflectance of the background, and of other plant seedlings growing in the vicinity of the M. bancana seedlings. We hypothesize that the M. bancana seedlings would have a similar color reflectance to the background and to be distinctive compared to adult M. bancana plants and to seedlings of other plant taxa.
Methods
The common mahang (Macaranga bancana) is a small to medium sized tree that grows up to 20 to 23 m high (). The bark is pale-brown to whitish, mottled. The large leaves are characteristically 3-lobed (). At the base of each leaf stalk is a pair of dark purple stipules, and many white starch grains are found on their abaxial surface. Young leaves are reddish-brown, densely covered by trichomes (). It is common in Peninsular Thailand, Peninsular Malaysia, Sumatra and Borneo. Macaranga bancana can be found in a broad range of habitats. The species can thrive on both clay and sandy soils, although the preference is toward the latter due to the water retention in clay. As such, M. bancana can be found in places where non-pioneer species could not thrive, such as wet gullies along roadsides, alluvial forest areas, and the margins of degraded peat swamp forests.
Figure 1 (a) Adult M. bancana.(b) Close up of a normal adult Macaranga Bancana. It is easily identified based on its trilobe shape.
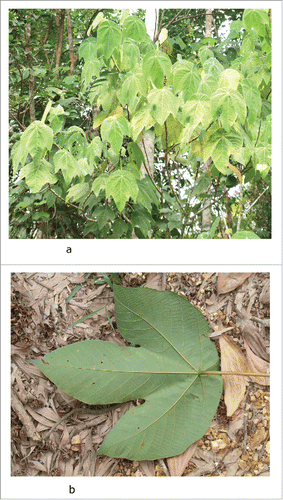
Figure 2. M. bancana seedling. There is evidence of insect herbivory at some of the seedlings based on the damages on the young leaves. The seedlings, however are surrounded by other syntopic green seedlings that stands out among the background.

We gathered the M. bancana samples from the Gunung Ledang National Park, Johore, Malaysia (N2 20.802 E102 38.181). Spectral measurements follow protocols suggested by Fadzly et al.Citation4. We took spectral readings from 30 individual M. bancana seedlings (less than 20 cm in height). We also took spectral readings from 30 adult M. bancana (more than 20 cm in height). These include both saplings and full grown adult trees. The spectral measurements for both groups were similar, therefore we grouped them together. The background reflectance was also measured. We took the average of 15 spectral measurements of the background which consists mostly of dead leaves, woody debris, soil and rocks. We sorted the spectral results for the background into 2 categories, which were background soil and background dry leaves. The categories infer to the main component. For comparison purposes, we took the spectral measurements of other types of seedlings growing nearby the M. bancana seedlings. We took 30 individual measurements of the other species seedlings (less than 20 cm in height). However, most of the seedlings were unidentifiable. Although we could not clearly identify each of the seedlings, their general appearance and coloration suggests that they are very different from the M. bancana seedlings.
Spectral measurements were conducted using OceanOptics Jazz Portable Spectrophotometer with Tungsten lamp light source. Reflectance was measured as the proportion if a diffuse reflectance standard (Teflon coated white standard, OceanOptics). The distance between each object and the probe was fixed at 1 cm, with the angle of illumination and reflection fixed at 45° to reduce glare. Irradiance was measured using cosine corrected sensor and D65 (normal daylight) bulb as reference. Spectra were calculated at 5-nm intervals from 300 to 700 nm with SpectraSuite software (OceanOptics).
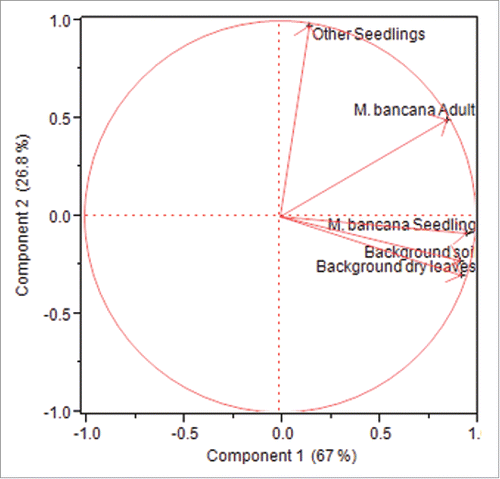
There are multiple options in analyzing the reflectance properties. Endler and MielkeCitation16 suggested using the simple color pattern measures related to photon capture of a known, specific receptor spectral sensitivity of the observer. Fadzly et al.Citation4 based their calculations on the eye receptor sensitivities of ostriches, as the closest proxy of the extinct Moa birds (the targeted herbivore in the study). Niu et. alCitation3 uses butterflies (as potential pollinators) to calculate discriminability among the 2 variants of Corydalis benecincta. However for this study, we analyzed the reflectance based on a slightly modified protocol described by Klooster et al.Citation2 The reason we did not choose a specific targeted observer, is because M. bancana is a pioneer species found in an open/disturbed areas in a tropical rainforest. In a tropical rain forest, there are various types of herbivores ranging from ungulates to small insects. There are no specific (monophagus) herbivores that exclusively target M. bancana. Principal Component Analysis has an advantage in this study because the method considers both hue and chroma in a simple graphical summary (see Klooster et al.Citation2). There might be differences based on different types of animal eye receptor sensitivities, but for this analysis, PCA transformation is sufficient to validate our results. A PCA analysis was performed with JMP 10 software package using raw spectral values sampled at 5 nm increments from the M. bancana seedlings, other species seedlings, adult M. bancana and the background (both soil and leaves).
Results
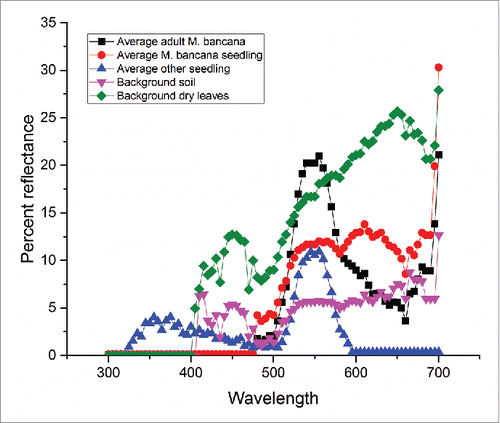
shows the reflectance analysis results. Based on the curves, Adult M. bancana and seedlings of other species have an almost similar reflected spectrum. Both showed a peak at around 550 nm, which is green in the visible light. The adult leaves give a higher reflected light intensity compared to that of the seedlings. The light reflected from M. bancana seedlings does not show a specific peak, and shows an increase from 500 nm to 650 nm, a combination between green-red in the visible color spectrum. For the background spectrum, soil is much darker/ lower intensity and shows a range of wavelengths ranging from 400 nm to 700 nm. Dry leaves also show similar colors, with a much higher intensity compared to the other variables. Although the spectrum of the background does suggest the appearance of blue, this is attributed to small stones and clay soil that are mixed in the background. The spectral analysis alone does show how the M. bancana seedlings are remarkably visually different from the adult stage and also from the other species seedlings coloration. The seedling's spectral reflectance closely resembles the soil background.
Figure 3. Spectral reflectance results. The adult Macaranga bancana and other seedlings shows similar pattern, whereas the seedlings Macaranga bancana are almost similar to the background.
As an additional analysis, our PCA analysis created 5 eigenvalues, with the first 2 factors account for 93.8 % of the variation. PC1 independently accounted for the majority of the variation at 67 % with PC2 representing the next highest value at 26.8 %. shows the eigenvector values for PC1 and PC2. Other seedlings and M. bancana adults are clustered independently of each other in the ordination space. In contrast M. bancana seedlings, background soil and background dry leaves are clustered together within the same region ().
Table 1. Eigenvectors For PCA analysis.
Discussion
Despite the research attention that had been given M. bancana adults, very little is known about the seedlings. Experienced park rangers at the sampling site had acknowledged, that even though they could recognize an adult M. bancana, they have never bothered to inspect and examine the seedlings. When asked to identify the seedlings, the rangers failed to locate them. After further information and an example picture were given, only then the rangers managed to spot the seedlings.
Based on our spectral results, the reflectance curve shows that the adults and other seedling colors are similar, whereas the M. bancana seedlings are more similar to the background color. The average spectral properties of both adults M. bancana and other seedlings are also ordinary, that is mostly within the standard error of the leaves of syntopic species. Their colors are consistent with the reflectance properties of the primary pigments involved in photosynthesis (chlorophyll a and b). These two pigments have peak absorption values above and below the peak in reflectance of adult leaves at 540–550 nm. Further PCA results also support our hypothesis that the M. bancana seedlings are more similar to the background color (both soil and fallen leaves).
There are multiple hypotheses of why leaves are red colored. The red coloration could be attributed to the presence of anthocyanin pigments. These pigments produce colors ranging from brown to red or in some cases as a trade-off between the amount of chlorophyll in the leaves. The most common hypothesis is protection against photoinhibition. Excessive bright light condition could reduce photosynthesis efficiency and could possibly lead permanent damage to the chloroplasts, cells and tissues.Citation17 However, Krause et al.Citation18 argued that for tropical plants (young leaves), the main component against photoinhibition are xanthophylls, α-carotene, and zeaxanthin. This argument is supported by Dominy et al.,Citation19 in stating that if anthocyanins are involved in photoprotection, there should be more tropical trees with red leaves at the canopy level, compared to the understory.
Another hypothesis that might explain red colorations of young leaves in the tropics is that red color makes the leaves cryptic to insect herbivores.Citation19 Most of the insect herbivores lack the spectral sensitivity in the red region (600– 700 nm wavelength).Citation19,20 Tellez et al.Citation21 suggests that the anthocyanins protect the young leaves from fungal damage. The amount of anthocyanins in the leaves could also produce a bitter, inedible taste that could possibly repel grazing herbivores.Citation22
The cryptic red colors of M. bancana seedlings and its subsequent change during the adult stage resembles the Pseudopanax crassifolius and Elaocarpus hookerianus that changes throughout its ontogeny.Citation1,4 It can be speculated that M. bancana changes its cryptic coloration after development of the hollow stem and food bodies that will attract ants. The ants in turn will provide defense for the plants from browsing herbivores. This cryptic coloration is quite peculiar and undocumented strategy for M. bancana. As a pioneer species, fast resprouting and growth would be an advantage.
There is a caveat of having red coloration, Archetti et al.Citation23 argued that red colored leaves is counterproductive as it needs extra costs of pigment synthesis, resource loss and decreases the photosynthesis rate. Therefore, the red leaves are thought of as a handicap signal to deter colonizing insect herbivores. It must be noted that this hypothesis is a based on temperate trees, rather than tropical. A more recent study by Chen and Huang,Citation24 suggests that through phylogenetic analysis, young red leaves have less mechanical protection (in the form of enhanced cuticle, epidermis and trichomes) compared to the green young leaves. Menzies et alCitation25 demonstrated that the red leaves of the Pseudowintera colorata, has a higher concentration of antifeedant chemical components, incurring less herbivory damage and reduced leaf eating infestation from insects. Hughes and Lev-Yadun Citation26 discusses the numerous potential ecological and physiological of having red colored leaf margin. Various existing hypothesis supports one another, depending on the environment or the locality of the species. For further example, delayed leaf greening is a useful trait for tropical trees. A delayed greening or red-colored young leaf strategy in seedlings have been linked to higher survival and growth rate further influencing the species composition in later stages.Citation27
For the M. bancana, the cryptic coloration serves it's purpose based on the species ecological niche. As a pioneer species, the seedlings are quite unprotected in an open area. Being cryptic definitely increases the survival rate, as browsing herbivores would be more inclined toward a conspicuous green foliage. In a tropical forest and disturbed areas, there are numerous herbivores, from deer, cows and to the smallest leaf cutter insects. We do note that there was evidence of insect herbivory for some of the M. bancana seedlings sample, however, it was in a smaller amount and limited to a certain ant infested treefall gap area. Whether the red colors work to deter insect herbivory for M. bancana seedlings still remain open to experimentation and discussion. As far as for humans' eye vision, without prior information and a trained eye, the seedlings remain quite inconspicuous. We would like to suggest that the cryptic coloration of M. bancana seedlings as an additional useful defensive strategy for a pioneering plant species.
Disclosure of potential conflict of interest
The authors would like to declare that we have no conflict of interest and financial obligations concerning the publication of this data and manuscript. The authors also adhere to the code and conduct of scientific publication.
Acknowledgments
The authors would like to thank the Perbadanan Taman Negara Johor and Gunung Ledang National Park rangers for their help and guidance. We would like to thank Prof Simcha Lev-yadun and another anonymous reviewer for their comments in improving the manuscripts. We would also like to thank Nor Amira Rahman for helping out with the data analysis.
Funding
This study is supported by RU USM Grant 1001/PBIO/811329.
References
- Fadzly N, Burns KC. Hiding from the Ghost of Herbivory Past: Evidence for Crypsis in an Insular Tree Species. Int J Plant Sci 2010; 171:828-33; http://dx.doi.org/10.1086/654850
- Klooster MR, Clark DL, Culley TM. Cryptic bracts facilitate herbivore avoidance in the mycoheterotrophic plant Monotropsis odorata (Ericaceae). Am J Bot 2009; 96:2197-205; PMID:21622335; http://dx.doi.org/10.3732/ajb.0900124
- Niu Y, Chen G, Peng DL, Song B, Yang Y, Li ZM, Sun H. Grey leaves in an alpine plant: a cryptic colouration to avoid attack? New Phytol 2014; 203(3):953-63.
- Fadzly N, Jack C, Schaefer HM, Burns KC. Ontogenetic colour changes in an insular tree species: signalling to extinct browsing birds? New Phytologist 2009; 184:495-501; PMID:19674327; http://dx.doi.org/10.1111/j.1469-8137.2009.02926.x
- Kavanagh PH, Shaw RC, Burns KC. Potential aposematism in an insular tree species: are signals dishonest early in ontogeny? Biol J Linnean Society 2016; http://dx.doi.org/10.1111/bjj.12785
- La Rocca N, Pupillo P, Puppi G, Rascio N. Erythronium dens-canis L. (Liliaceae): An unusual case of change of leaf mottling. Plant Physiology and Biochemistry 2014; 74:108-17; PMID:24291157; http://dx.doi.org/10.1016/j.plaphy.2013.11.005
- Stone BC. Protective coloration of young leaves in certain Malaysian palms. Biotropica 1979; 11:126-; http://dx.doi.org/10.2307/2387788
- Kursar TA, Coley PD. Delayed greening in tropical leaves: an antiherbivore defense? Biotropica 1992; 2(4):256-62; http://dx.doi.org/10.2307/2388520
- Coley PD, Aide TM. Red coloration of tropical young leaves: a possible antifungal defence? J Tropical Ecology 1989; 5:293-300; http://dx.doi.org/10.1017/S0266467400003667
- Lev-Yadun S, Dafni A, Flaishman MA, Inbar M, Izhaki I, Katzir G, Ne'eman G. Plant coloration undermines herbivorous insect camouflage. BioEssays 2004; 26:1126-30; PMID:15382135; http://dx.doi.org/10.1002/bies.20112
- Lev-Yadun S. Aposematic (Warning) Coloration in Plants. In: Baluska F, ed. Plant-Environment Interactions, Signaling and Communication in Plants. Berlin Heidelberg: Springer-Verlag, 2009.
- Davies SJ, Ashton PS. Phenology and fecundity in 11 sympatric pioneer species of Macaranga (Euphorbiaceae) in Borneo. Am J Bot 1999; 86:1786-95; PMID:10602770; http://dx.doi.org/10.2307/2656675
- Itioka, T. (2005). Diversity of anti-herbivore defenses in Macaranga. In Pollination Ecology and the Rain Forest (pp. 158-171). Springer New York.
- Murase K, Itioka T, Nomura M, Yamane S. Intraspecific variation in the status of ant symbiosis on a myrmecophyte, Macaranga bancana, between primary and secondary forests in Borneo. Population Ecology 2003; 45:221-6; http://dx.doi.org/10.1007/s10144-003-0158-4
- Itino T, Itioka T, Hatada A, Hamid AA. Effects of food rewards offered by ant-plant Macaranga on the colony size of ants. Ecological Res 2001; 16:775-86; http://dx.doi.org/10.1046/j.1440-1703.2001.00433.x
- Endler JA, Mielke PW. Comparing entire colour patterns as birds see them. Biol J Linnean Society 2005; 86:405-31; http://dx.doi.org/10.1111/j.1095-8312.2005.00540.x
- Lee DW, Gould KS. Why leaves turn red. American Scientist 2002; 90:524; http://dx.doi.org/10.1511/2002.39.794
- Krause GH, Virgo A, Winter K. High susceptibility to photoinhibition of young leaves of tropical forest trees. Planta 1995; 197:583-91; http://dx.doi.org/10.1007/BF00191564
- Dominy NJ, Lucas PW, Ramsden LW, Riba‐Hernandez P, Stoner KE, Turner IM. Why are young leaves red? Oikos 2002; 98:163-76; http://dx.doi.org/10.1034/j.1600-0706.2002.980117.x
- Fadzly N, Burns KC. What weta want: colour preferences of a frugivorous insect. Arthropod-Plant Interactions 2010; 4:267-76; http://dx.doi.org/10.1007/s11829-010-9109-0
- Tellez P, Rojas E, Van Bael S. Red Coloration in Young Tropical Leaves Associated with Reduced Fungal Pathogen Damage. Biotropica 2016; 48:150-3; http://dx.doi.org/10.1111/btp.12303
- Harborne JB. The Chemical Basis of Plant Defense. London: CRC Press, 1991.
- Archetti M, Döring TF, Hagen SB, Hughes NM, Leather SR, Lee DW, Lev-Yadun S, Manetas Y, Ougham HJ, Schaberg PG. Unravelling the evolution of autumn colours: an interdisciplinary approach. Trends Ecol Evolution 2009; 24:166-73; PMID:19178979; http://dx.doi.org/10.1016/j.tree.2008.10.006
- Chen Y-Z, Huang S-Q. Red young leaves have less mechanical defence than green young leaves. Oikos 2013; 122:1035-41; http://dx.doi.org/10.1111/j.1600-0706.2012.20852.x
- Menzies IJ, Youard LW, Lord JM, Carpenter KL, Klink JW, Perry NB, Schaefer HM, Gould KS. Leaf colour polymorphisms: a balance between plant defence and photosynthesis. J Ecol 2016; 104:104-13; http://dx.doi.org/10.1111/1365-2745.12494
- Hughes NM, Lev-Yadun S. Red/purple leaf margin coloration: Potential ecological and physiological functions. Environ Exp Bot 2015; 119:27-39; http://dx.doi.org/10.1016/j.envexpbot.2015.05.015
- Queenborough SA, Metz MR, Valencia R, Wright SJ. Demographic consequences of chromatic leaf defence in tropical tree communities: do red young leaves increase growth and survival? Ann Bot 2013; 112:677-84; PMID:23881717; http://dx.doi.org/10.1093/aob/mct144