ABSTRACT
Nucleotide-Binding Site-Leucine-Rich Repeat (NBS-LRR) genes are the largest plant disease resistance (R) gene family, accounting for ∼80% of more than 140 cloned R genes. Recently, we systematically investigated NBS-LRR genes in 22 angiosperm genomes. By performing phylogenetic analysis of these genes in major angiosperm clades separately and as a whole, we gained strong evidence supporting that angiosperm NBS-LRR genes are derived from 3 anciently separated NBS-LRR classes: RPW8-NBS-LRR (RNL), TIR-NBS-LRR (TNL) and CC-NBS-LRR (CNL). A total of 23 ancestral NBS-LRR lineages gave rise to the current NBS-LRR diversity of angiosperm through dynamic expansions. Comparative analysis of RNL, TNL, and CNL classes revealed that while RNL genes evolved conservatively to maintain its role in defense signal transduction, the latter 2 classes underwent convergent recent expansions in various plant genomes. The revealed evolutionary pattern of angiosperm NBS-LRR genes reflects a long history of competition between plant and pathogen.
KEYWORDS:
Plant disease resistance (R) genes play crucial roles in plant defense against the invasions of various pathogens.Citation1 Currently, more than 140 R genes have been cloned from several angiosperms, among which ∼80% belong to the nucleotide-binding site-leucine-rich repeat (NBS-LRR) gene family. Benefits from the conserved NBS domain in NBS-LRR proteins, high-throughput bioinformatics methods for genome-wide identification of NBS-LRR genes have been developed,Citation2,3 with which dozens to hundreds of NBS-LRR genes have been identified from various angiosperm plant genomes.Citation4-9
Previous studies have divided angiosperm NBS-LRR genes into 2 separate classes, TIR-NBS-LRR (TNL) and non-TIR-NBS-LRR (nTNL), based on the presence or absence of a Toll/IL-1 receptor-like (TIR) domain at the N-terminal of translated proteins.Citation10,11 The nTNL genes are also called CC-NBS-LRR genes (CNL), which is based on a frequently detected coiled-coil (CC) domain at the N-terminal of nTNL proteins.Citation2 This early-proposed classification system has been largely followed in the past 10 y of NBS-LRR gene investigations. However, several recent studies have examined a group of NBS-LRR genes with a special RPW8 domain at the N terminus.Citation5,7,12 This group of RPW8-NBS-LRR (RNL) genes has not been found to be involved in detecting specific pathogens as most known TNL or CNL type R genes, but functions in signal transductions of disease resistances.Citation12,13 A controversy was then raised by comparing independent phylogenetic analysis, referring to whether RNL genes are derived from a CNL lineage recently or represent another ancient NBS-LRR class that sister to CNL and TNL genes.Citation2,5,7 To clarify this basic concept for NBS-LRR gene classification, our recent studyCitation6 performed large-scale phylogenetic analysis of NBS-LRR genes from 22 genomes across major angiosperm lineages. In combination with motif composition analysis of the NBS domain and intron structure and phase detection, our study provides strong evidence that RNL is an ancient NBS-LRR class that has been largely ignored by most previous studies.Citation6 Thus our results suggest that angiosperm NBS-LRR genes are composed of 3 anciently diverged classes, namely, TNL, CNL, and RNL.
Given the large number of NBS-LRR genes observed in modern angiosperms, it is interesting to explore how many ancestral lineages of the 3 NBS-LRR classes had diverged prior to the divergence of angiosperms, and how the current NBS-LRR profile in individual genomes evolved. These questions were further addressed in our study by tracing the ancient state of NBS-LRR genes at key divergence nodes of angiosperms step by step.Citation6 A total of 23 NBS-LRR lineages were finally recovered in the common ancestor of investigated angiosperms.Citation6 Further comparison of ancestral NBS-LRR lineages at the internal divergence nodes of angiosperm, including mesangiospermae (Ma), eudicots (Ed), monocots (Mo), asterids (As), and rosids (Ro), revealed that 3 NBS-LRR classes underwent distinct evolutionary patterns during angiosperm radiation.Citation6 The RNL gene retained a few copies during the entire evolutionary history of angiosperms, which well reflects its functional restriction for defense signal transduction.Citation6 In contrast, both TNL and CNL genes underwent intensive recent expansions.Citation6 Interestingly, the starting point of intensive expansions of both TNL and CNL genes from different angiosperm lineages were traced to the K-P boundary ∼66 million years ago.Citation6 The reported dramatic environment change and bloom of pathogenic fungi at this periodCitation14-17 allowed us to speculate that an increased selection pressure from pathogens might have driven the intensive and convergent expansions of TNL and CNL genes in various angiosperm lineages at this stage.Citation6
Although TNL and CNL genes exhibit convergent recent expansion, these have underwent completely different evolutionary processes during the first 100 million years evolution of angiosperm. While CNL gradually expanded its ancestral lineages from 14 to several dozens, TNL genes retained <10 copies during this period.Citation6 The absence of TNL genes in monocots has been a long-standing mystery of NBS-LRR gene evolution.Citation18 Recent studies revealed that TNL genes are also absent from the genome of a basal dicot Aquilegia coerulea and several genomes of lamiales in the asterid lineage.Citation12 Considering tens to hundreds of TNL genes present in many dicot genomes and the basal angiosperm Amborella trichopoda, it is difficult to imagine how such a large class of NBS-LRR genes has been completely deleted from different angiosperm lineages. The long-term contraction of TNL genes during the early evolution of angiosperm revealed in our studyCitation6 provides a possible explanation for this question. In this scenario, the deletion of a few TNL genes from some early diverged angiosperm lineages is not impossible.Citation6
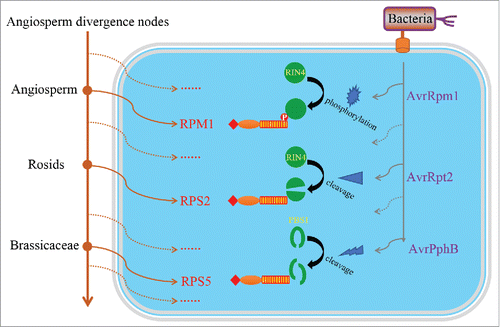
The established phylogenetic framework in our studyCitation6 not only provides new insights into the evolutionary history of 3 different NBS-LRR classes, but also enables us to determine the mechanism underlying the formation of the current NBS-LRR profile in a genome as well as the approximate origination time of functional R genes. Using Arabidopsis thaliana genome as an example, we revealed that the 5 known functional CNL genes originated from different angiosperm divergence nodes.Citation6 Three CNL genes (RPM1, RPS2, and RPS5) defending the same bacterium Pseudomonas syringae were traced to the divergence nodes of angiosperm, rosids, and brassicaceae, respectively.Citation6 These detect the infection of P. syringae by monitoring the modification of 2 host proteins by 3 different P. syringae effector proteins.Citation19-21 A proposed evolutionary history of these 3 R genes with distinct defending mechanismsCitation19-21 is presented in , which well reflects the long-term arms race between plant and pathogens.
Figure 1. The evolutionary history of 3 functional R genes against Pseudomonas syringae. To evade the activation of plant immune system, bacteria have evolved various effector proteins to modify or disrupt certain important host immune-related proteins. As an early-originating R gene, RPM1 specifically detects the phosphorylation of RIN4, which is caused by the P. syringae effector protein AvrRpm1. P. syringae then evolved another effector (AvrRpt2) to evade the activation of RPM1 by cleaving the RIN4 protein. In response, plants further evolved RPS2 to monitor the cleavage of RIN4 and activate the immune system. On the other hand, P. syringae evolved AvrPphB to target another host protein PBS1 for cleavage, which is subsequently recognized by a later evolved R gene RPS5. Solid circles indicate divergence nodes in angiosperm clade. Solid arrows indicate the origin of R genes (in brown) or bacterial effector proteins (in gray), whereas dashed arrows indicate unknown R genes or effector proteins that developed during its evolutionary history.
Understanding the evolutionary history of NBS-LRR genes is fundamental to the proper utilization and artificial modification of this large R gene family. Our recent studyCitation6 provides a fundamental framework of angiosperm NBS-LRR genes, which we believe would be helpful in generating further insights into the structural and functional evolution of NBS-LRR genes, as well as for mining R gene resources from different plant species.
Disclosure of potential confllicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National Natural Science Foundation of China (31400201, 31470327, and 31570217), and the National Postdoctoral Science Foundation of China (2013M540435 and 2014T70503).
References
- DeYoung BJ, Innes RW. Plant NBS-LRR proteins in pathogen sensing and host defense. Nat Immunol 2006; 7:1243-9; PMID:17110940; http://dx.doi.org/10.1038/ni1410
- Meyers BC, Kozik A, Griego A, Kuang H, Michelmore RW. Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 2003; 15:809-34; PMID:12671079; http://dx.doi.org/10.1105/tpc.009308
- Shao ZQ, Zhang YM, Wang B, Chen JQ. Computational identification of microRNA-targeted nucleotide-binding site-leucine-rich repeat genes in plants. Bio-protocol 2015; 5.
- Lin X, Zhang Y, Kuang H, Chen J. Frequent loss of lineages and deficient duplications accounted for low copy number of disease resistance genes in Cucurbitaceae. BMC Genomics 2013; 14:335; PMID:23682795; http://dx.doi.org/10.1186/1471-2164-14-335
- Shao ZQ, Zhang YM, Hang YY, Xue JY, Zhou GC, Wu P, Wu XY, Wu XZ, Wang Q, Wang B, et al. Long-term evolution of nucleotide-binding site-leucine-rich repeat genes: understanding gained from and beyond the legume family. Plant Physiol 2014; 166:217-34; PMID:25052854; http://dx.doi.org/10.1104/pp.114.243626
- Shao ZQ, Xue JY, Wu P, Zhang YM, Wu Y, Hang YY, et al. Large-scale analyses of angiosperm nucleotide-binding site-leucine-rich repeat (NBS-LRR) genes reveal three anciently diverged classes with distinct evolutionary patterns. Plant Physiol 2016; 170:15; http://dx.doi.org/10.1104/pp.15.01487
- Zhang YM, Shao ZQ, Wang Q, Hang YY, Xue JY, Wang B, Chen JQ. Uncovering the dynamic evolution of nucleotide-binding site-leucine-rich repeat (NBS-LRR) genes in Brassicaceae. J Integr Plant Biol 2016; 58:13; PMID:25926337; http://dx.doi.org/10.1111/jipb.12365
- Wei C, Chen J, Kuang H. Dramatic number variation of R genes in Solanaceae species accounted for by a few R gene subfamilies. PLoS One 2016; 11:e0148708; PMID:26849045; http://dx.doi.org/10.1371/journal.pone.0148708
- Wu P, Shao ZQ, Wu XZ, Wang Q, Wang B, Chen JQ, Hang YY, Xue JY. Loss/retention and evolution of NBS-encoding genes upon whole genome triplication of Brassica rapa. Gene 2014; 540:54-61; PMID:24576745; http://dx.doi.org/10.1016/j.gene.2014.01.082
- Meyers BC, Dickerman AW, Michelmore RW, Sivaramakrishnan S, Sobral BW, Young ND. Plant disease resistance genes encode members of an ancient and diverse protein family within the nucleotide-binding superfamily. Plant J 1999; 20:317-32; PMID:10571892; http://dx.doi.org/10.1046/j.1365-313X.1999.00606.x
- Cannon SB, Zhu HY, Baumgarten AM, Spangler R, May G, Cook DR, Young ND. Diversity, distribution, and ancient taxonomic relationships within the TIR and non-TIR NBS-LRR resistance gene subfamilies. J Mol Evol 2002; 54:548-62; PMID:11956693; http://dx.doi.org/10.1007/s0023901-0057-2
- Collier SM, Hamel LP, Moffett P. Cell death mediated by the N-terminal domains of a unique and highly conserved class of NB-LRR protein. Mol Plant Microbe Interact 2011; 24:918-31; PMID:21501087; http://dx.doi.org/10.1094/MPMI-03-11-0050
- Bonardi V, Tang S, Stallmann A, Roberts M, Cherkis K, Dangl JL. Expanded functions for a family of plant intracellular immune receptors beyond specific recognition of pathogen effectors. Proc Natl Acad Sci USA 2011; 108:16463-8; PMID:21911370; http://dx.doi.org/10.1073/pnas.1113726108
- Ma LJ, Geiser DM, Proctor RH, Rooney AP, O'Donnell K, Trail F, Gardiner DM, Manners JM, Kazan K. Fusarium pathogenomics. Annu Rev Microbiol 2013; 67:399-416; PMID:24024636; http://dx.doi.org/10.1146/annurev-micro-092412-155650
- Slippers B, Boissin E, Phillips AJ, Groenewald JZ, Lombard L, Wingfield MJ, Postma A, Burgess T, Crous PW. Phylogenetic lineages in the Botryosphaeriales: a systematic and evolutionary framework. Stud Mycol 2013; 76:31-49; PMID:24302789; http://dx.doi.org/10.3114/sim0020
- Blonder B, Royer DL, Johnson KR, Miller I, Enquist BJ. Plant ecological strategies shift across the Cretaceous-Paleogene boundary. PLoS Biol 2014; 12:e1001949; PMID:25225914; http://dx.doi.org/10.1371/journal.pbio.1001949
- Hedges SB, Marin J, Suleski M, Paymer M, Kumar S. Tree of life reveals clock-like speciation and diversification. Mol Biol Evol 2015; 32:835-45; PMID:25739733; http://dx.doi.org/10.1093/molbev/msv037
- Meyers BC, Kaushik S, Nandety RS. Evolving disease resistance genes. Curr Opin Plant Biol 2005; 8:129-34; PMID:15752991; http://dx.doi.org/10.1016/j.pbi.2005.01.002
- Mackey D, Holt BF, 3rd, Wiig A, Dangl JL. RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell 2002; 108:743-54; PMID:11955429; http://dx.doi.org/10.1016/S0092-8674(02)00661-X
- Bent AF, Kunkel BN, Dahlbeck D, Brown KL, Schmidt R, Giraudat J, Leung J, Staskawicz BJ. RPS2 of Arabid opsis thaliana: a leucine-rich repeat class of plant disease resistance genes. Science 1994; 265:1856-60; PMID:8091210; http://dx.doi.org/10.1126/science.8091210
- Shao F, Golstein C, Ade J, Stoutemyer M, Dixon JE, Innes RW. Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science 2003; 301:1230-3; PMID:12947197; http://dx.doi.org/10.1126/science.1085671
