ABSTRACT
Mounting evidence suggests that microtubules play important roles in several aspects of autophagy in mammalian cells, such as autophagosome biogenesis, autophagosome trafficking and autolysosome formation. However, little research attention has been paid to the engagement of microtubules in plant autophagy. Recently, we reported novel findings in Nicotiana benthamiana that disruption of microtubules reduces autophagosome formation during upregulation of macroautophagy and triggers a specific type of chloroplast autophagy (SEX chlorophagy), which is closely associated with the starch-excess phenotype of leaves. These findings reveal important functional links between microtubules, autophagy and leaf starch degradation in plants.
Abbreviations
| ATG | = | autophagy related |
| chlorophagy | = | chloroplast autophagy |
| DPE1 | = | disproportionating enzyme 1 |
| pGlcT | = | plastidic sugar transporters |
| PtdIns3K | = | phosphatidylinositol 3-kinase |
| Sex | = | starch-excess |
| TUB | = | β-tubulin |
| TUA | = | α-tubulin |
Microtubules, the key components of the cytoskeleton in eukaryotic cells, are tubular filaments composed of α- and β-tubulin heterodimers. In mammalian cells, they are involved in membrane rearrangement events and provide tracks for long-range transport of membrane-bound vesicles and organelles.Citation1 Their importance in macroautophagy (hereafter referred to as autophagy unless otherwise specified), a well-known self-degradation process characterized by the formation of double-membrane autophagosomes,Citation2 has also been highlighted by recent studies on cytoskeleton-autophagy interactions in mammalian systems.Citation3-5 In contrast, little is known about the role of microtubules in plant autophagy, apart from a few reports on colocalizations of microtubules and autophagy related proteins, ATG8 and JOKA2, in planta.Citation6,7
One β-tubulin protein, TUB8, was identified as an interacting factor of ATG6 (autophagy-related protein 6) in our recent work.Citation8 Further analysis showed interactions of ATG6 with several other α-/β-tubulin proteins, including TUA6, TUB1 and TUB6, and its colocalizations with microtubules. Consistent with this, BECN1, the mammalian ortholog of ATG6, had been reported to be recruited to microtubules by 2 complexes containing dynein light chain 1 (DYNLL1/LC8) and AMBRA1 or BCL2L11/Bim to regulate autophagy onset.Citation9,10 To figure out the exact role of microtubules in plant autophagy, we disrupted the cortical microtubule arrays in N. benthamiana leaves via silencing of tubulin genes or treatment with microtubule-depolymerizing agents, and investigated the effect of microtubule disassembly on autophagy in 2 types of autophagic processes: nocturnal autophagy and oxidation-induced autophagy. The former refers to a kind of basal autophagy occurring in leaves at night which undergoes an upregulation before midnight and a downregulation by dawn, while the latter is artificially induced by methyl viologen treatment in leaves.Citation8,11 Our results showed that, during upregulation of both autophagic processes, the formation of CFP-ATG8f-labeled autophagic structures was significantly reduced in tubulin-silenced or antimicrotubule herbicides-treated mesophyll cells. However, autophagosome formation at low baseline in those microtubule-disorganized cells was not hampered.Citation8 These results suggest that an intact microtubule network may not be crucial element of autophagosome formation but indeed facilitate autophagosomes biogenesis during autophagy upregulation. Similar roles of microtubules in autophagosome formation had been reported in mammalian cells.Citation12-14 Considering that the class III phosphatidylinositol 3-kinase (PtdIns3K ) complex containing Vps30/BECN1, the orthologs of ATG6 in yeast and mammals, is essential for the nucleation step of autophagosome formation by producing PtdIns3P and recruiting PtdIns3P-binding autophagy proteins,Citation15,16 the association of ATG6 with microtubules in plants probably makes the recruitment more efficient, which further promotes the biogenesis of autophagosome.
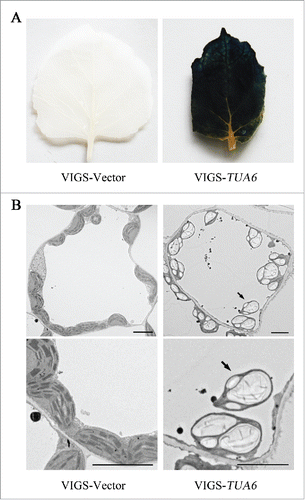
In the ultrastructural study of TUB8-silenced leaves, we observed that lots of large starch granules accumulated in chloroplasts of TUB8-silenced mesophyll cells even at the end of night-time metabolism, which led to malformation, degeneration and degradation of chloroplast. Time lapse imaging of TUB8-silenced cells showed Brownian motion of chloroplasts or chloroplastic structures within the central vacuole, which further supports the vacuolar degradation of chloroplast.Citation8 Similar starch-excess (sex) phenotype and chloroplast degradation were observed in TUA6-silenced leaves (), as well as antimicrotubule herbicides-treated plants,Citation8 confirming that the cause of these phenotypes is microtubule disruption. Reducing starch contents by co-silencing of TUB8 with APS1, an essential gene involved in starch synthesis encoding the small subunit 1 of ADP-glucose pyrophosphorylase, can alleviate the vacuolar degradation of chloroplast, suggesting that the level of starch accumulation is an important contributor to this process. Therefore, we called this form of self-eating process for removing misshapen starchy chloroplast in the central vacuole ‘starch excess-associated chloroplast autophagy’ (hereafter short for SEX chlorophagy). Interestingly, SEX chlorophagy proceeds well even in cells where TUB8 was co-silenced with ATG5 or ATG6 or ATG7, suggesting that these ATG genes are dispensable for SEX chlorophagy. This finding, together with the observation of partially suppressed macroautophagic activity in microtubule-disorganized cells, indicates that the occurrence of SEX chlorophagy is independent of canonical autophagosome-mediated activities. In fact, we have not observed any chloroplast engulfed in membranous vesicles either in the cytoplasm or the vacuole under transmission electron microscopy. How the starchy chloroplasts enter vacuole is currently unknown. To clarify this, 2 main questions remain to be answered in the future. First, is the degradation process selective? Assuming that it is selective, how the cells sense the special signals for chloroplast degradation and recognize the target precisely? Second, given that macroautophagy is dispensable for SEX chlorophagy, does microautophagy engage in this process, and, if so, are starchy chloroplasts taken into vacuole by direct invagination of vacuolar membrane and which genes are required for the process? Actually, all these mentioned concerns are not limited to SEX chlorophagy, but also true for other reported vacuolar degradation processes of chloroplasts induced by senescence, gene mutations or virus infection.Citation17-20 It is additionally worth mentioning that, although similar chloroplast degradation associated with high starch levels has been observed in Arabidopsis mex1 mutant lacking maltose transporter or its double mutants, dpe1 mex1 and pglct mex1, lacking disproportionating enzyme 1 (DPE1) or plastidic sugar transporters (pGlcT),Citation18,21 we are uncertain whether it occurs via the same way with SEX chlorophagy described here. On the one hand, while the aberrant chloroplasts shown in mex1 or its double mutants are usually lacking regular boundary and the degradative structures within vacuolar compartment are almost chloroplast remnants, most of the starchy chloroplasts appearing in the cytoplasm or the vacuole of microtubule-disorganized cells are whole structures. On the other hand, the ATG dependency of chloroplast degradation in mex1 mutant has not been elucidated yet.
Figure 1. Silencing of TUA6 in N. benthamiana leads to starch overaccumulation and occurrence of SEX chlorophagy. (A) Iodine staining of leaves detached from TUA6-silenced and nonsilenced plants at the end of night. (B) Ultrastructural analysis shows occurrence of SEX chlorophagy in TUA6-silenced plants. Leaf samples used for TEM sectioning were taken from plants that had just finished nocturnal metabolism at 3 weeks post-agroinfiltration for silencing of TUA6. Arrows refer to the vacuole-localized sex chloroplasts. Scale bars: 5 μm.
Starch assays related to TUB8 individually silenced or TUB8/APS1 co-silenced plants demonstrated that the extreme sex phenotype was caused by a blockage of starch turnover, suggesting that microtubule integrity is necessary for leaf starch degradation.Citation8 Obviously, microtubules per se do not participate in the degradation process. What's the potential links between 2 seemingly unrelated things? As far as we know, 2 pathways involved in leaf starch degradation have been reported: one is the primary, plastidial pathway in which starch granules are converted into neutral sugars inside chloroplast;Citation22 the other one is the autophagic pathway described in our previous work that contributes to the degradation of small starch granule-like structures.Citation11,23 Therefore, the suppressed nighttime autophagic activities in microtubule-disorganized cells bridge microtubules with the defects in autophagic leaf starch degradation. Additionally, the classical plastidial pathway of starch degradation seems to be also blocked in microtubule-disorganized cells, otherwise a much more severe sex phenotype than ATG6-silenced leaves would not have been observed in TUB8-silenced leaves.Citation8 However, the mechanism linking microtubules and the plastidial starch degradation is currently unknown.
Taken together, our findings demonstrate that an intact microtubule cytoskeleton is important for efficient autophagosome formation and leaf starch degradation in plants. Also, we report a new type of chlorophagy, SEX chlorophagy, which is closely associated with starch overaccumulation.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National Basic Research Program of China (2011CB910100) and China Postdoctoral Science Foundation (2014M550048). Y. W. was supported in part by the Postdoctoral Fellowship of Tsinghua-Peking Joint Center for Life Sciences.
References
- Rogers SL, Gelfand VI. Membrane trafficking, organelle transport, and the cytoskeleton. Curr Opin Cell Biol 2000; 12:57-62; PMID:10679352; http://dx.doi.org/10.1016/S0955-0674(99)00057-5
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 2004; 6:463-77; PMID:15068787; http://dx.doi.org/10.1016/S1534-5807(04)00099-1
- Monastyrska I, Rieter E, Klionsky DJ, Reggiori F. Multiple roles of the cytoskeleton in autophagy. Biol Rev Camb Philos Soc 2009; 84:431-48; PMID:19659885; http://dx.doi.org/10.1111/j.1469-185X.2009.00082.x
- Mackeh R, Perdiz D, Lorin S, Codogno P, Pous C. Autophagy and microtubules - new story, old players. J Cell Sci 2013; 126:1071-80; PMID:23620510; http://dx.doi.org/10.1242/jcs.115626
- Mostowy S. Multiple roles of the cytoskeleton in bacterial autophagy. PLoS Pathog 2014; 10:e1004409; PMID:25412422; http://dx.doi.org/10.1371/journal.ppat.1004409
- Ketelaar T, Voss C, Dimmock SA, Thumm M, Hussey PJ. Arabidopsis homologues of the autophagy protein Atg8 are a novel family of microtubule binding proteins. FEBS Lett 2004; 567:302-6; PMID:15178341; http://dx.doi.org/10.1016/j.febslet.2004.04.088
- Zientara-Rytter K, Sirko A. Selective autophagy receptor Joka2 co-localizes with cytoskeleton in plant cells. Plant Signal Behav 2014; 9:e28523; PMID:24705105; http://dx.doi.org/10.4161/psb.28523
- Wang Y, Zheng X, Yu B, Han S, Guo J, Tang H, Yu AY, Deng H, Hong Y, Liu Y. Disruption of microtubules in plants suppresses macroautophagy and triggers starch excess-associated chloroplast autophagy. Autophagy 2015; 11:2259-74; PMID:26566764; http://dx.doi.org/10.1080/15548627.2015.1113365
- Di Bartolomeo S, Corazzari M, Nazio F, Oliverio S, Lisi G, Antonioli M, Pagliarini V, Matteoni S, Fuoco C, Giunta L, et al. The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J Cell Biol 2010; 191:155-68; PMID:20921139; http://dx.doi.org/10.1083/jcb.201002100
- Luo S, Garcia-Arencibia M, Zhao R, Puri C, Toh PP, Sadiq O, Rubinsztein DC. Bim inhibits autophagy by recruiting Beclin 1 to microtubules. Mol Cell 2012; 47:359-70; PMID:22742832; http://dx.doi.org/10.1016/j.molcel.2012.05.040
- Wang Y, Yu B, Zhao J, Guo J, Li Y, Han S, Huang L, Du Y, Hong Y, Tang D, et al. Autophagy contributes to leaf starch degradation. Plant Cell 2013; 25:1383-99; PMID:23564204; http://dx.doi.org/10.1105/tpc.112.108993
- Fass E, Shvets E, Degani I, Hirschberg K, Elazar Z. Microtubules support production of starvation-induced autophagosomes but not their targeting and fusion with lysosomes. J Biol Chem 2006; 281:36303-16; PMID:16963441; http://dx.doi.org/10.1074/jbc.M607031200
- Kochl R, Hu XW, Chan EY, Tooze SA. Microtubules facilitate autophagosome formation and fusion of autophagosomes with endosomes. Traffic 2006; 7:129-45; PMID:16420522; http://dx.doi.org/10.1111/j.1600-0854.2005.00368.x
- Xie R, Nguyen S, McKeehan WL, Liu L. Acetylated microtubules are required for fusion of autophagosomes with lysosomes. BMC Cell Biol 2010; 11:89; PMID:21092184; http://dx.doi.org/10.1186/1471-2121-11-89
- Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol 2009; 10:458-67; PMID:19491929; http://dx.doi.org/10.1038/nrm2708
- Lamb CA, Yoshimori T, Tooze SA. The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol 2013; 14:759-74; PMID:24201109; http://dx.doi.org/10.1038/nrm3696
- Niwa Y, Kato T, Tabata S, Seki M, Kobayashi M, Shinozaki K, Moriyasu Y. Disposal of chloroplasts with abnormal function into the vacuole in Arabidopsis thaliana cotyledon cells. Protoplasma 2004; 223:229-32; PMID:15221529; http://dx.doi.org/10.1007/s00709-004-0037-7
- Stettler M, Eicke S, Mettler T, Messerli G, Hortensteiner S, Zeeman SC. Blocking the metabolism of starch breakdown products in Arabidopsis leaves triggers chloroplast degradation. Mol Plant 2009; 2:1233-46; PMID:19946617; http://dx.doi.org/10.1093/mp/ssp093
- Wada S, Ishida H, Izumi M, Yoshimoto K, Ohsumi Y, Mae T, Makino A. Autophagy plays a role in chloroplast degradation during senescence in individually darkened leaves. Plant Physiol 2009; 149:885-93; PMID:19074627; http://dx.doi.org/10.1104/pp.108.130013
- Ishida H, Wada S. Autophagy of whole and partial chloroplasts in individually darkened leaves: a unique system in plants? Autophagy 2009; 5:736-7; PMID:19395861; http://dx.doi.org/10.4161/auto.5.5.8568
- Cho MH, Lim H, Shin DH, Jeon JS, Bhoo SH, Park YI, Hahn TR. Role of the plastidic glucose translocator in the export of starch degradation products from the chloroplasts in Arabidopsis thaliana. New Phytol 2011; 190:101-12; PMID:21175634; http://dx.doi.org/10.1111/j.1469-8137.2010.03580.x
- Streb S, Zeeman SC. Starch metabolism in Arabidopsis. Arabidopsis Book 2012; 10:e0160; PMID:23393426; http://dx.doi.org/10.1199/tab.0160
- Wang Y, Liu Y. Autophagic degradation of leaf starch in plants. Autophagy 2013; 9:1247-8; PMID:23722252; http://dx.doi.org/10.4161/auto.25176
