ABSTRACT
Heterotrimeric G-proteins, consisting of Gα, Gβ and Gγ subunits, are important signal transducers in eukaryotes. In plants, G-protein-mediated signaling contributes to defense against a range of fungal and bacterial pathogens. Here we studied response of G-protein-deficient mutants to ssRNA viruses representing 2 different families: Cucumber mosaic virus (CMV) (Bromoviridae) and Turnip mosaic virus (TuMV) (Potyviridae). We found that development of spreading necrosis on infected plants was suppressed in the Gβ-deficient mutant (agb1-2) compared to wild type and Gα-deficient mutant (gpa1-4). In accordance, ion leakage caused by viral infection was also significantly reduced in agb1-2 compared to wild type and gpa1-4. Nevertheless, both viruses replicated better in agb1-2 plants, while gpa1-4 was similar to wild type. Analysis of pathogenesis-related genes showed that Gβ negatively regulated salicylic acid, jasmonic acid and abscisic acid marker genes during CMV and TuMV infections. Interestingly, analysis of salicylic acid deficient transgenic plants indicated that salicylic acid did not affect resistance against these viruses and did not influence the Gβ-mediated defense response. We conclude that heterotrimeric G-proteins play a positive role in defense against viral pathogens probably by promoting cell death.
Introduction
Heterotrimeric G-proteins, consisting of 3 subunits Gα, Gβ and Gγ, are essential eukaryotic signaling molecules, amplifying signals perceived by receptors at the plasma membrane and passing it to cytoplasmic effectors.Citation1-5 In plants, heterotrimeric G-proteins are involved in multiple biological processes that influence plant development and stress responses.Citation4-23 Importantly, Gβ and Gγ subunits participate in disease resistance against necrotrophic and hemi-biotrophic pathogens.Citation5,9,14-18,24-26 To the best of our knowledge G-protein role in defense against viral pathogens never been studied. Most probably, G-proteins mediate several various defense related pathways responding to different types of pathogens.Citation9,15,18,26 Reasonable amount of experimental evidence supports a hypothesis that G-protein-mediated resistance against hemi-biotrophic fungi and bacteria may be associated with receptor-like kinases (RLKs) and the defense mechanism is probably based on activation of programmed cell death (PCD).Citation15,16,24,27-29 Recently we established that heterotrimeric G-proteins physically interact with 3 defense-related receptor-like kinases, in particular with BRI1-associated kinase 1, BAK1.Citation30 It has been reported that viral RNA induces responses that relied on RLKs.Citation31 BAK1, for instance, has been shown to play a role in resistance against 3 different RNA viruses.Citation32 We predicted that G-proteins might be a part of this signaling pathway and play a role in defense against viral pathogens.
Salicylic acid (SA)-mediated defense system is a well-known contributor to PCD as well.Citation33-35 It is highly essential for resistance to biotrophic pathogens.Citation36,37 Infection by compatible viruses induces SA defense-related gene expression.Citation32,38-40 In particular, pathogenesis-related 1 (PR1) transcript was greatly increased upon treatment with several compatible viruses.Citation41 Upregulation of this gene reliably indicates activation of SA-mediated defense pathway. However, contribution of this pathway in resistance against viruses remains unclear.Citation42-46 It has been shown that G-protein mediated defense and PCD responses were independent of SA.Citation15,17,20,28 Therefore these 2 pathways might contribute additively or synergistically to PCD and/or provide resistance against viruses.
Cucumber mosaic virus (CMV) and Turnip mosaic virus (TuMV) belong to 2 different families of positive strand, single stranded (ss) RNA viruses. They both infect multiple plant species, including Arabidopsis and represent the largest group of viral plant pathogens.Citation47-49 Apart from being a significant agricultural threats, these viruses represent well-studied systems for plant-virus interaction, with optimized experimental conditions.
In this paper we report the positive role of heterotrimeric G-proteins in defense against 2 ssRNA viruses, CMV and TuMV. Our results demonstrate a number of alterations to defense response caused by null mutation in AGB1 gene. We suggest that G-protein-mediated resistance against viral pathogens involves augment of cell death.
Results
Differential response of G-protein deficient mutants to TuMV and CMV infection
Since G-proteins have never before been associated with defense against viruses, we chose to start with well characterized viruses – Cucumber mosaic virus (CMV) and Turnip mosaic virus (TuMV). To establish involvement of G-proteins in resistance against these viruses we inoculated 5-week-old A. thaliana null mutants lacking Gα (gpa1-4) or Gβ (agb1-2) subunits as well as wild type (WT) Columbia-0 (Col-0) plants. To characterize disease progression and defense response we observed several symptomatic characteristics specific for virus-infected plants: discoloration of rosette leaves, leaf curling and arrest of inflorescence growth. All genotypes were affected by both viruses, and developed visible disease symptoms. However, we observed clear differences in severity and dynamics of disease progression in different genotypes. The onset of disease was indicated by leaf discoloration, which started as obvious chlorotic patches (mottling) on rosette leaves of inoculated plants, while mock-inoculated plants remained uniformly green. The symptoms developed faster and were more severe in CMV-inoculated plants with appearance at 8 days post inoculation (dpi), while TuMV caused first apparent symptoms at 11 dpi. Further observation revealed that chlorosis was progressive and eventually distinct necrosis developed at 16 dpi and 21 dpi on CMV- and TuMV-infected plants, respectively (). Importantly, chlorotic mottling was perceptibly more developed in WT and gpa1-4 compared to agb1-2 (). The apparent difference between genotypes was observed in respect to necrotic areas, which were not detected upon visual inspection in agb1-2 plants. To study necrosis development, we stained representative leaves from each genotype with trypan blue. Mock-inoculated WT leaves used as a control displayed no necrotic areas, WT and gpa1-4 leaves infected with virus showed clear patches of deeply stained dead tissue, while only small separated dots were observed on infected agb1-2 leaves (). This demonstrates that G-protein mutant agb1-2, but not gpa1-4, was impaired in response to viral infection in terms of induced cell death.
Figure 1. AGB1, but not GPA1, knockout suppresses development of the cell death symptoms induced by CMV or TuMV infection. (A) Development of chlorosis and necrosis on designated genotypes infected with CMV or TuMV 16 and 21 dpi, respectively. Necrosis is obviously less developed in agb1-2 and NahG agb1-2 compared to WT (Col-0), gpa1-4 or NahG. (B) Detection of dead cells using trypan blue staining. Lower intensity of blue staining in agb1-2 and NahG agb1 compared to WT (Col-0), gpa1-4 and NahG indicates suppression of induced cell death by agb1 mutation.
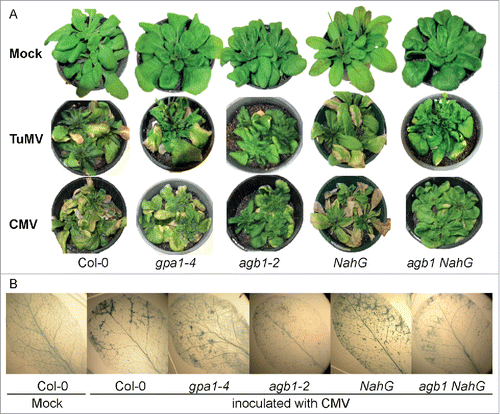
It has been reported that plants expressing the salicylate hydroxylase gene, NahG, which are deficient in SA, showed wild type-like resistance to CMV,Citation42,50 indicating that basal resistance to CMV is not SA-dependent. To test the involvement of SA-mediated signaling in resistance against CMV in our experimental system and to study its interaction with AGB1-mediated response, we inoculated SA-depleted, NahG transgenic plants, agb1-2 mutant and agb1-2/NahG plants along with WT plants with CMV. The NahG plants showed no difference with WT in symptoms (), in accordance with previous reports.Citation42,50 At the same time, agb1/NahG plants were very similar in symptom development and severity to agb1-2 plants (). Analysis of trypan blue staining of infected leaves displayed the WT necrosis development on NahG plants and suppressed necrosis on agb1-2/NahG leaves (). Therefore we conclude that endogenous SA does not affect the AGB1-mediated response.
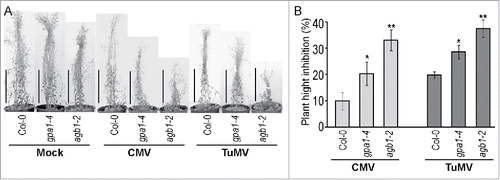
Stunted inflorescence is another distinct symptom caused by infection with CMV or TuMV. Therefore, we evaluated suppression of inflorescence elongation by comparing plant's height of mock- and virus-inoculated plants 6 weeks after inoculation. All genotypes displayed shorter inflorescence in infected plants compared to mock-inoculated controls (). Interestingly, despite an earlier onset of chlorosis and necrosis caused by CMV, the inflorescence growth was stronger inhibited by TuMV. Moreover, both viruses suppressed inflorescence growth more severely in agb1-2 mutant compared to WT and gpa1-4. The effect of the viral infection on inflorescence length expressed as a percentage of mock-inoculated plant height is shown on . The shown difference between WT and agb1-2 was statistically significant (Student's t-test P < 0.001, n = 10). Notably, infected gpa1-4 plants also exhibited more stunted inflorescence compared to WT, although to a lesser extent than agb1-2 (). Student's t-test supported statistical significance of the observed differences between WT and gpa1-4, as well as gpa1-4 and agb1-2 (P < 0.05, n = 10).
Figure 2. Plant hight inhibition by viral infection was more prominent in agb1-2 mutants. (A) Several representative plants of listed genotypes were mock-inoculated or infected with CMV or TuMV. Plants were analyzed and photographed 5 weeks after inoculation. (B) Quantification of the inflorescence growth suppression by viral infection. Values indicate mean ratio of (infected plant height) / (average mock-inoculated plant height) ± SE. Asterisks indicate significant differences from wild type Col-0 (Student's t-test, * P < 0.05, ** P < 0.01). Experiment was repeated 3 times with similar results.
In addition, non-quantifiable symptoms such as leaf curling and loss of primary inflorescence dominance manifested as multiple stunted secondary inflorescences emerging from the base of the rosette were more pronounced in infected agb1-2 plants compared to WT or gpa1-4. The summary of the symptom analysis is: i) chorosis, and necrosis were less developed in agb1-2 compared to WT and gpa1-4, while leaf curling and inflorescence impairment were significantly more developed in agb1-2; ii) inflorescence growth was also inhibited in gpa1-4 plants, although to a lesser extent than in agb1-2; and iii) endogenous SA did not affect resistance to CMV and TuMV and did not interfere with AGB1-mediated responses.
Gβ mutant displayed reduced cell death-related responses
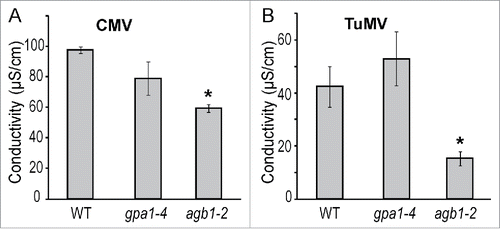
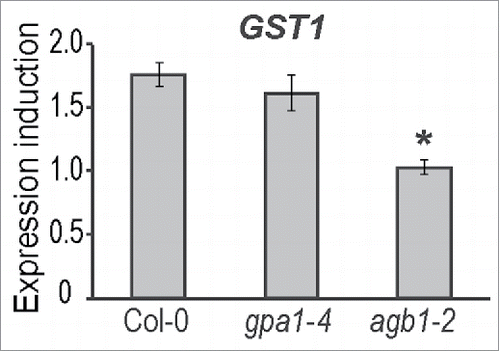
We observed that chlorotic and necrotic lesions caused by CMV and TuMV infection were significantly less developed in the agb1-2 mutant compared to WT and gpa1-4 plants. Therefore, we assumed that agb1-2 mutant may be impaired in other cell death-related responses. One of the well-known method for determining cell death in plants is assessing ion leakage from plant tissue.Citation51 We quantified the ion leakage in virus-inoculated leaves for the 3 genotypes. Leaf samples from CMV- and TuMV-infected plants were analyzed at 1 and 3 dpi, respectively. Conductivity tests showed higher levels of ion leakage in WT and gpa1-4 compared to agb1-2 (), which is consistent with the reduced level of necrotic symptoms observed in agb1-2 leaves. One of the indicators of cell death at gene expression level is glutathione S-transferase 1, GST1. We evaluated GST1 expression level in CMV-infected plants 24 hours after inoculation and compared it with mock-inoculated control. CMV did not induce GST1 expression in agb1-2 plants, while significant almost 2-fold induction was observed in WT and gpa1-4 (). These experiments confirmed the impairment of cell death responses in agb1-2 mutant.
Figure 3. Cell death related ion leakage response to viral infection was compromised in AGB1 deficient plants. Conductivity test was performed on plants of designated genotypes challenged with (A) CMV or (B) TuMV. The ion leakage was evaluated 24 or 72 hours after infection, respectively. Values indicate mean conductivity ± SE. Asterisks indicate significant differences from wild type Col-0 (Student's t-test, * P < 0.05).
Systemic virus accumulation was increased in Gβ deficient mutant
The reduced necrosis and impaired cell death observed in agb1-2 mutant implies that it may be less restrictive to viral replication and/or systemic movement within the plant. To evaluate viral replication and long-distance movement, we measured accumulation of viral coat protein (CP) mRNA in un-inoculated leaves of CMV- and TuMV-infected plants. Both viral CP and its RNA have been successfully used for quantification of viral particles and genomes in infected plants.Citation52-54 Two-3 fully expanded leaves of 5-week-old plants of the 3 genotypes: WT, gpa1-4 and agb1-2 were inoculated with CMV or TuMV. Seven days after inoculation, total RNA was extracted from adjacent un-inoculated leaves. cDNA was produced by reverse transcription and subjected to quantitative PCR using CP gene primers specific for each virus. As shown in , agb1-2 mutant accumulated significantly higher levels of CP mRNA than WT or gpa1-4 plants for both CMV and TuMV.
Figure 4. Expression induction of GST1 in plants infected with CMV. The expression was evaluated with RT-qPCR using SAND expression for normalization. Values on the graph represent means of the 3 ratios from 3 independent replicates, error bars show standard error of the mean. Asterisks indicate statistically significant differences from wild type Col-0 (Student's t-test, P < 0.05).
CMV- and TuMV-induced PR gene expression is enhanced in agb1-2 mutant
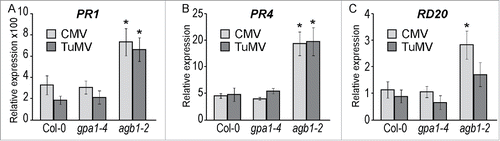
To test if pathogenesis-related hormonal pathways governed by salicylic acid (SA) and jasmonic acid (JA) or abscisic acid (ABA) are affected by G-protein mediated signaling, we evaluated expression induction of the several marker genes: PR1 for SA; PR4 for JA and RD20 for ABA. Plants of the 3 genotypes were inoculated with CMV, TuMV or mock-inoculated. RNA was extracted one week after inoculation. Relative gene expression was determined by quantitative PCR. Induction levels were calculated as a ratio of virus-inoculated to mock-inoculated gene expression. Distinctively high induction of PR1 expression was detected in response to both viruses in all 3 genotypes. Importantly, in agb1-2 the induction was significantly higher compared to WT and gpa1-4 (). CMV infection resulted in PR1 levels increased approximately 300-fold in WT and gpa1-4, but about 700-fold in agb1-2 mutant. Similarly, TuMV induced PR1 levels 200-fold in WT and gpa1-4, while in agb1-2 the increase was about 650-fold (). Both viruses were considerably less effective inducing JA and ABA reporter genes, PR4 and RD20, respectively. While PR4 gene was induced about 5-fold in WT and gpa1-4 and up to 20-fold in agb1-2, RD20 gene was not induced in WT and gpa1-4 and only up to 3-fold induction was observed in agb1-2 (). All described differences were statistically significant (Student's t-test, P < 0.05).
Figure 5. Viral RNA accumulation was higher in AGB1 deficient plants. Five-week-old Arabidopsis plants were inoculated with CMV or TuMV and assessed after 7 days; total RNA was extracted from leaves neighboring the ones used for infection. Viral RNA accumulation was quantified by RT-qPCR targeting the viral coat protein (CP) gene and using Arabidopsis SAND gene for normalization. (A) CMV CP gene accumulation. (B) TuMV CP gene accumulation. Values on the graph represent means of 4 independent biological replicates, error bars show standard error of the mean. Asterisks indicate statistically significant differences from wild type Col-0 (Student's t-test, P < 0.05).
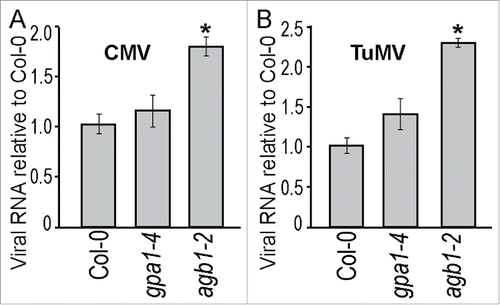
Figure 6. Expression of pathogenesis-related genes upon viral infection. Relative expression of (A) PR1, (B) PR4, and (C) RD20 evaluated by RT-qPCR at 7 dpi. Values were normalized with Arabidopsis SAND gene. Values on the graph represent means of the 3 independent biological replicates, and error bars show standard error of the mean. Asterisks indicate statistically significant differences from wild type Col-0 (Student's t-test, P < 0.05).
Discussion
G-proteins have been shown to play an important role in defense against bacterial and fungal pathogens.Citation6,9,14,16-18,26 Here we provided the first evidence that G-protein-mediated signaling is also involved in resistance against viral pathogens. We found that agb1-2 mutant lacking Gβ subunit was more susceptible to 2 ssRNA viruses, CMV and TuMV compared to wild type, Col-0. In contrast, the Gα deficient mutant gpa1-4 was similar to WT in symptom development and virus accumulation. These observations are reminiscent of previous reports in which Gβ deficient mutant is hypersensitive to fungal and bacterial pathogens, while Gα mutant displays sensitivity similar to wild type.Citation9,14,18,24,26,28 Therefore, we conclude that the Gα subunit is not involved in the Gβ-mediated defense pathway. Interestingly, recently it was shown that Gβγ actually partnering with extra-large G-proteins (XLG2 and XLG3) to facilitate resistance against fungal and bacterial pathogens.Citation15 Importantly, the apparent similarity in susceptibility of the agb1-2 mutant to 3 different groups of pathogens, bacteria, fungi and viruses, suggests a universal defense mechanism controlled by Gβ subunits. Similarly increased virus accumulation was caused by mutations in receptor-like kinase BAK1 gene.Citation32 The bak1-4 and bak1-5 mutants were found to be more susceptible to 3 different RNA viruses Oilseed rape mosaic virus (ORMV), Turnip crinkle virus (TCV) and Tobacco mosaic virus (TMV).Citation32 Recently we have demonstrated direct physical interaction between BAK1 and G-protein subunits, Gα, Gγ1 and Gγ2.Citation30 Here, we observed that Gα did not contribute to the resistance against CMV or TuMV, while Gβ did. Gβ forms compulsory dimers with Gγ1 and Gγ2 subunits and does not function independently.Citation55,56 Therefore it is reasonable to speculate that Gβγ dimer might function together with BAK1 forming a signal transduction module, which initiates cell death response upon attack of various pathogens. Existence of such a module and its universal defense role has been substantiated by 2 recent reports.Citation27,31 Liang an co-authors have demonstrated that G-proteins, namely non-canonical Gα, XLG2, the Gβ, AGB1, and the 2 Gγ subunits, AGG1 and AGG2 directly bind Flagellin Sensitive2 (FLS2)-BIK1 receptor complex and are required for flagellin-induced immune responses.Citation27 This work provided a mechanistic evidence for G-protein role in PTI. While the other group established that viral double stranded RNAs can act as pathogen-associated molecular pattern (PAMP), which induces specific signaling cascades leading to PAMP-triggered immunity PTI induction. In this picture G-proteins represent one of jigsaw-puzzle piece further connecting elements of complex defense network.
The first and most striking observation we made was a superficial contradiction of visual symptom severity. On one hand, agb1-2 rosette leaves showed little chlorosis and negligible amount of spreading necrosis, compared to WT leaves that were covered densely with yellow and brown areas. On the other hand, number of curled leaves and severely stunted inflorescences prevailed on agb1-2 plants, while WT was significantly less affected in this respect. Usually, symptom severity correlates positively with pathogen progression and advance of the disease. Nevertheless, some symptoms, although indicative of the pathogen presence, are in fact a result of host defense systems activation. For example, formation of the necrotic lesions might be a result of programmed cell death, developed by plants to withstand attacks of obligate biotrophs and viruses.Citation47,57 These pathogens could not procreate in dead tissue; therefore, infection is usually contained (in case of incompatible interaction) or at least delayed. For these reasons we evaluated virus resistance by assessment of different types of symptoms and confirmed virus accumulation and movement by relative quantification of the viral RNA present in infected plants. Collectively, our data indicate that agb1-2 mutant was more susceptible to both viruses compared to WT, despite showing less necrosis. The logical explanation for the latter is a participation of AGB1 in cell death development. This hypothesis was supported by reduced ion leakage and GST1 expression in agb1-2 mutant. AGB1 involvement in cell death has been suggested through its association with BIR1 (BAK1-interacting receptor like kinase 1).Citation24,28,58 Arabidopsis bir1-1 mutant shows constitutive activation of defense response genes and generalized cell death resulting in early lethality at the seedling stage.Citation58 It was demonstrated that agb1 bir1 double mutants displayed suppressed cell death observed in bir1.Citation28 Noteworthy, Gα deficiency was unable to reverse the bir1 phenotype. Considering this data, we concluded that failure of the agb1-2 mutant to perform cell death initiated by the host plants as a defense mechanism against viruses resulted in increased susceptibility.
Our gene expression analysis signified that PR1, a marker gene for SA-regulated immune response, was drastically induced by virus infection in agb1-2 mutant, suggesting a role for SA in AGB1-mediated resistance. SA plays an important role in plant defense and is essential for the initiation of cell death.Citation34,59-61 Importantly, external application of SA has been shown to increase resistance against TuMV in Chinese cabbage Citation62 and against CMV in tobacco and Arabidopsis by inhibiting viral replication and viral systemic movement.Citation45 On the other hand, plants expressing the bacterial NahG, and thus, producing negligible levels of endogenous SA, displayed wild-type resistance levels to the virus as reported previously Citation42,50,63 and confirmed by our experiments. This set of data suggests no role for endogenous SA in resistance against CMV. Our epistasis analysis also showed that endogenous SA did not affect AGB1-mediated response. This conclusion is in agreement with previous observations that AGB1- and SA-mediated pathways contribute independently to complementation of the bir1-1 phenotype caused by enhanced cell death Citation28 and to defense against fungal pathogens.Citation15,17,20 Excessive upregulation of PR1 expression in agb1-2 mutant therefore, could be explained as a negative control of SA signaling by G-proteins.
We speculate that upon recognition of a virus, by yet unknown receptor(s) the signal transducing element, consisting of the heterotrimeric G-proteins, initiates the defense mechanism based on cell death. Thereby cell death deficient agb1-2 plants inevitably become predisposed to faster viral replication and/or movement yielding higher levels of viral particles. The initial failure to contain the viral infection results in the increased severity of secondary disease symptoms (leaf curling and inflorescence inhibition) and stronger induction of the PR genes. We hypothesize that heterotrimeric G-protein signaling mediated through Gβ, but not canonical Gα, subunit contributes in defense response by promoting cell death-associated mechanisms. Future studies will clarify functional interactions between signaling elements of this plant defense pathway and reveal detailed mechanism of action.
Materials and methods
Plant material and experimental conditions
All A. thaliana mutant lines (agb1-2, gpa1-4, NahG, NahG agb1) were in the Columbia-0 background. All mutants including double knockouts NahG agb1 had been previously described.Citation18,20 Plants were grown in University of California potting soil mixture in Percival growth chamber with 10hr-light/14hr-darkness photoperiod at 21°C/23°C with a relative humidity of 75% and a light intensity of 90 μmol m−2s−1.
Virus maintenance and plant inoculation
Turnip mosaic virus (TuMV) strain 2080 was originally isolated from wild radish in Victoria (Australia) and subsequently propagated in Pak Choy. Cucumber mosaic virus (CMV) strain 207 was originally isolated from tomato in Queensland (Australia) and belongs to subgroup IA, which causes severe symptoms on Nicotiana species Citation64; it is closely related to the well-characterized isolate Fny (Owen et al., 1990), which causes severe symptoms in A. thaliana Col-0 (Zhang et al., 2006; Wang et al., 2010). Freeze-dried leaf samples stored at −20°C were re-activated by mechanical inoculation of 5-weeks-old Nicotiana benthamiana leaves. The infected plant was maintained at 23°C with a 12/12 hours light/dark cycle. Arabidopsis inoculation was described previously.Citation64,65 Briefly, 2-3 N. benthamiana leaves with obvious symptoms were removed and grounded in 5 ml of 10 mM sodium phosphate buffer (pH 7.4) containing 20 mM sodium sulfite producing the inoculation solution. Three or 4 fully expanded leaves of 5-week-old Arabidopsis were wounded by gently rubbing with carborundum block, rub-inoculated with the inoculation solution and rinsed briefly with tap water. Control plants were treated with the buffer only. Inoculated and control plants were kept in growth chamber at 21°C/23°C with a 12/12 hours light/dark cycle with light intensity of 90 μmol m−2s−1 and 75% relative humidity in a Percival growth chamber.
Extraction and quantitative PCR with reverse transcription analysis
Total RNA was extracted from leaves as described.Citation66 RNA samples were treated with DNaseI (Invitrogen) as per manufacturer's instruction. Reverse transcription was performed with SuperScript III reverse transcriptase kit (Invitrogen) following manufacturer's instructions. Plant gene expression and viral CP RNA levels were evaluated by quantitative PCR with reverse transcription (RT-qPCR) using FastStart Essentials DNA Green Master (Roche Applied Sciences) following manufacturer's protocol in a 96 Light Cycler system (Roche). Primers for the selected genes used in gene expression analysis and for CMV and TuMV CP genes were: PR4-F TGCTACATCCAAATCCAAGCCT, PR4-R CGGCAAGTGTTTAAGGGTGAAG Citation20; PR1-F AAGAGGCAACTGCAGACTCA, PR1-R TCTCGCTAACCCACATGTTC,Citation20 RD20-F CCGAAGGAAGGTATGTCCCAG, RD20-R TTCGATTTCCCTCGGTTACATTC Citation20; CMV-F TGAGAAAGTACGCCGTCCTC, CMV-R GATGTGGGAATGCGTTGGTG; TuMV-F AAGACCGACCATACATGCCAC, TuMV-R CCTCTCTCGCACGTATTGGAG; GST1-F TAATAAAAGTGGCGATGACC, GST1-R ACATTCAAATCAAACACTCG.Citation67 Gene expression was analyzed with Light cycler 96 SW (version 1.1) software and normalized to reference gene, SAND (primers for RT-qPCR: SAND-F GTTGGGTCACACCAGATTTTG, SAND-R GCTCCTTGCAAGAACACTTCA).Citation68 Data was analyzed using method suggested in.Citation69
Necrotic tissue staining
Detection of necrosis caused by cell death was carried out with trypan blue staining as described previously.Citation70 Briefly, leaves were boiled in lactophenol trypan blue solution (20 ml phenol, 20 ml lactic acid, 40 ml glycerol, 20 ml water and 0.05% trypan blue mix and added 200 ml 96% ethanol) for 2 min. Leaves were distained in chloral hydrate overnight and viewed under a Carl Zeiss Axio Scope A1 microscope equipped with interference or phase-contrast optics.
Quantification of ion leakage
The ion leakage was evaluated as described previously.Citation71 In brief, 3 days after inoculation, 5 leaves were collected from infected plants in 3 replicates. Six millimeter in diameter leave discs were cut out and placed in a tube with 5 ml of deionized distilled water and shaken on a rotary shaker at 100 rpm at room temperature for 30 min. Conductivity of the resulted water solution was measured at designated time intervals with conductivity meter Orion 130 (Boston).
Disclosure of potential confllicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank AusAID for scholarship funding for EB. We thank John Thomas for virus isolates from the Queensland Department of Agriculture and Fisheries plant virus collection.
References
- Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem 1987; 56:615-49; PMID:3113327; http://dx.doi.org/10.1146/annurev.bi.56.070187.003151
- Neer EJ. Heterotrimeric G proteins: organizers of transmembrane signals. Cell 1995; 80:249-57; PMID:7834744; http://dx.doi.org/10.1016/0092-8674(95)90407-7
- Neves SR, Ram PT, Iyengar R. G protein pathways. Science 2002; 296:1636-9; PMID:12040175; http://dx.doi.org/10.1126/science.1071550
- Perfus-Barbeoch L, Jones AM, Assmann SM. Plant heterotrimeric G protein function: insights from Arabidopsis and rice mutants. Curr Opin Plant Biol 2004; 7:719-31; PMID:15491922; http://dx.doi.org/10.1016/j.pbi.2004.09.013
- Urano D, Chen JG, Botella JR, Jones AM. Heterotrimeric G protein signalling in the plant kingdom. Open Biol 2013; 3:120186; PMID:23536550; http://dx.doi.org/10.1098/rsob.120186
- Assmann SM. G protein regulation of disease resistance during infection of rice with rice blast fungus. Sci STKE 2005; 2005:cm13; PMID:16291770; http://dx.doi.org/10.1126/stke.3102005cm13
- Chakravorty D, Botella JR. Over-expression of a truncated Arabidopsis thaliana heterotrimeric G protein g subunit results in a phenotype similar to a and b subunit knockouts. Gene 2007; 393:163-70; PMID:17383830; http://dx.doi.org/10.1016/j.gene.2007.02.008
- Chakravorty D, Trusov Y, Zhang W, Acharya BR, Sheahan MB, McCurdy DW, Assmann SM, Botella JR. An atypical heterotrimeric G-protein g-subunit is involved in guard cell K+-channel regulation and morphological development in Arabidopsis thaliana. Plant J 2011; 67:840-51; PMID:21575088; http://dx.doi.org/10.1111/j.1365-313X.2011.04638.x
- Delgado-Cerezo M, Sanchez-Rodriguez C, Escudero V, Miedes E, Fernandez PV, Jorda L, Hernández-Blanco C, Sánchez-Vallet A, Bednarek P, Schulze-Lefert P, et al. Arabidopsis heterotrimeric G-protein regulates cell wall defense and resistance to necrotrophic fungi. Mol Plant 2012; 5:98-114; PMID:21980142; http://dx.doi.org/10.1093/mp/ssr082
- Ding L, Pandey S, Assmann SM. Arabidopsis extra-large G proteins (XLGs) regulate root morphogenesis. Plant J 2008; 53:248-63; PMID:17999646; http://dx.doi.org/10.1111/j.1365-313X.2007.03335.x
- Johnston CA, Taylor JP, Gao Y, Kimple AJ, Grigston JC, Chen JG, Siderovski DP, Jones AM, Willard FS. GTPase acceleration as the rate-limiting step in Arabidopsis G protein-coupled sugar signaling. Proc Natl Acad Sci USA 2007; 104:17317-22; PMID:17951432; http://dx.doi.org/10.1073/pnas.0704751104
- Jones JC, Temple BR, Jones AM, Dohlman HG. Functional reconstitution of an atypical G protein heterotrimer and regulator of G protein signaling protein (RGS1) from Arabidopsis thaliana. J Biol Chem 2011; 286:13143-50; PMID:21325279; http://dx.doi.org/10.1074/jbc.M110.190355
- Lease KA, Wen JQ, Li J, Doke JT, Liscum E, Walker JC. A mutant Arabidopsis heterotrimeric G-protein β subunit affects leaf, flower, and fruit development. Plant Cell 2001; 13:2631-41; PMID:11752377
- Llorente F, Alonso-Blanco C, Sanchez-Rodriguez C, Jorda L, Molina A. ERECTA receptor-like kinase and heterotrimeric G protein from Arabidopsis are required for resistance to the necrotrophic fungus Plectosphaerella cucumerina. Plant J 2005; 43:165-80; PMID:15998304; http://dx.doi.org/10.1111/j.1365-313X.2005.02440.x
- Maruta N, Trusov Y, Brenya E, Parekh U, Botella JR. Membrane-localized extra-large G proteins and Gbg of the heterotrimeric G proteins form functional complexes engaged in plant immunity in Arabidopsis. Plant Physiol 2015; 167:1004-16; PMID:25588736; http://dx.doi.org/10.1104/pp.114.255703
- Torres MA, Morales J, Sanchez-Rodriguez C, Molina A, Dangl J. Functional interplay between Arabidopsis NADPH oxidases and heterotrimeric G protein. Mol Plant-Microbe Interactions 2013; 26(6):686-94; PMID:23441575
- Trusov Y, Botella J. New faces in plant innate immunity: heterotrimeric G proteins. J Plant Biochem Biotechnol 2012; 21:40-7; http://dx.doi.org/10.1007/s13562-012-0140-3
- Trusov Y, Rookes JE, Chakravorty D, Armour D, Schenk PM, Botella JR. Heterotrimeric G proteins facilitate Arabidopsis resistance to necrotrophic pathogens and are involved in jasmonate signaling. Plant Physiol 2006; 140:210-20; PMID:16339801; http://dx.doi.org/10.1104/pp.105.069625
- Trusov Y, Rookes JE, Tilbrook K, Chakravorty D, Mason MG, Anderson D, Chen JG, Jones AM, Botella JR. Heterotrimeric G protein gamma subunits provide functional selectivity in G β gamma dimer signaling in Arabidopsis. Plant 2007; 19:1235-50; PMID:17468261; http://dx.doi.org/10.1105/tpc.107.050096
- Trusov Y, Sewelam N, Rookes JE, Kunkel M, Nowak E, Schenk PM, Botella JR. Heterotrimeric G proteins-mediated resistance to necrotrophic pathogens includes mechanisms independent of salicylic acid-, jasmonic acid/ethylene- and abscisic acid-mediated defense signaling. Plant J 2009; 58:69-81; PMID:19054360; http://dx.doi.org/10.1111/j.1365-313X.2008.03755.x
- Trusov Y, Zhang W, Assmann SM, Botella JR. Gg1 + Gg2 not equal to Gb: heterotrimeric G protein Gg-deficient mutants do not recapitulate all phenotypes of Gb-deficient mutants. Plant Physiol 2008; 147:636-49; PMID:18441222; http://dx.doi.org/10.1104/pp.108.117655
- Warpeha KM, Lateef SS, Lapik Y, Anderson M, Lee BS, Kaufman LS. G-protein-coupled receptor 1, G-protein Galpha-subunit 1, and prephenate dehydratase 1 are required for blue light-induced production of phenylalanine in etiolated Arabidopsis. Plant Physiol 2006; 140:844-55; PMID:16415218; http://dx.doi.org/10.1104/pp.105.071282
- Subramaniam G, Trusov Y, Lopez-Encina C, Hayashi S, Batley J, Botella JR. Type B Heterotrimeric G Protein gamma-Subunit Regulates Auxin and ABA signaling in tomato. Plant Physiol 2016; 170:1117-34; PMID:26668332; http://dx.doi.org/10.1104/pp.15.01675
- Ishikawa A. The Arabidopsis G-protein β-subunit is required for defense response against Agrobacterium tumefaciens. Biosci Biotechnol Biochem 2009; 73:47-52; PMID:19129659; http://dx.doi.org/10.1271/bbb.80449
- Lee S, Rojas CM, Ishiga Y, Pandey S, Mysore KS. Arabidopsis heterotrimeric G-proteins play a critical role in host and nonhost resistance against Pseudomonas syringae pathogens. PloS One 2013; 8:e82445; PMID:24349286; http://dx.doi.org/10.1371/journal.pone.0082445
- Trusov Y, Jorda L, Molina A, Botella JR. G Proteins and plant innate immunity. In: Sye AL, ed. Signal Commun Plants. Berlin: Springer, 2010; 221-50; http://dx.doi.org/10.1007/978-3-642-03524-1_12
- Liang X, Ding P, Lian K, Wang J, Ma M, Li L, Li L, Li M, Zhang X, Chen S, et al. Arabidopsis heterotrimeric G proteins regulate immunity by directly coupling to the FLS2 receptor. Elife 2016; 5; PMID:27043937; http://dx.doi.org/10.7554/eLife.13568
- Liu J, Ding P, Sun T, Nitta Y, Dong O, Huang X, Yang W, Li X, Botella JR, Zhang Y. Heterotrimeric G proteins serve as a converging point in plant defense signaling activated by multiple receptor-like kinases. Plant Physiol 2013; 161:2146-58; PMID:23424249; http://dx.doi.org/10.1104/pp.112.212431
- Trusov Y, Botella JR. Plant G-Proteins Come of Age: Breaking the Bond with Animal Models. Frontiers Chem 2016; 4:24; PMID:27252940; http://dx.doi.org/10.3389/fchem.2016.00024
- Aranda-Sicilia MN, Trusov Y, Maruta N, Chakravorty D, Zhang Y, Botella JR. Heterotrimeric G proteins interact with defense-related receptor-like kinases in Arabidopsis. J Plant Physiol 2015; 188:44-8; PMID:26414709; http://dx.doi.org/10.1016/j.jplph.2015.09.005
- Niehl A, Wyrsch I, Boller T, Heinlein M. Double-stranded RNAs induce a pattern-triggered immune signaling pathway in plants. N Phytologist 2016; 211(3):1008-19; PMID:27030513; http://dx.doi.org/10.1111/nph.13944
- Korner CJ, Klauser D, Niehl A, Dominguez-Ferreras A, Chinchilla D, Boller T, Heinlein M, Hann DR. The immunity regulator BAK1 contributes to resistance against diverse RNA viruses. Mol Plant-Microbe Interactions 2013; 26:1271-80; PMID:23902263; http://dx.doi.org/10.1094/MPMI-06-13-0179-R
- Alvarez ME. Salicylic acid in the machinery of hypersensitive cell death and disease resistance. Plant Mol Biol 2000; 44:429-42; PMID:11199399; http://dx.doi.org/10.1023/A:1026561029533
- Coll NS, Epple P, Dangl JL. Programmed cell death in the plant immune system. Cell Death Differ 2011; 18:1247-56; PMID:21475301; http://dx.doi.org/10.1038/cdd.2011.37
- Yoshimoto K. Plant autophagy puts the brakes on cell death by controlling salicylic acid signaling. Autophagy 2010; 6:192-3; PMID:20023431; http://dx.doi.org/10.4161/auto.6.1.10843
- Glazebrook J, Mitra R, Wang L. Signaling networks controlling, disease resistance responses in arabidopsis. Vitro Cell Dev Biol-Animal 2006; 42:11A-A; http://dx.doi.org/10.1007/Bf02668780
- Jones JD, Dangl JL. The plant immune system. Nature 2006; 444:323-9; PMID:17108957; http://dx.doi.org/10.1038/nature05286
- Carr JP, Lewsey MG, Palukaitis P. Chapter 3 - Signaling in induced resistance. In: John PC, Gad L, eds. Advances in virus research. Academic Press, 2010; 57-121; PMID: 20965072; http://dx.doi.org/10.1016/s0065-3527(10)76003-6
- Love AJ, Geri C, Laird J, Carr C, Yun BW, Loake GJ, Tada Y, Sadanandom A, Milner JJ. Cauliflower mosaic virus protein P6 inhibits signaling responses to salicylic acid and regulates innate immunity. PloS One 2012; 7:e47535; PMID:23071821; http://dx.doi.org/10.1371/journal.pone.0047535
- Love AJ, Laval V, Geri C, Laird J, Tomos AD, Hooks MA, Milner JJ. Components of Arabidopsis defense- and ethylene-signaling pathways regulate susceptibility to Cauliflower mosaic virus by restricting long-distance movement. Mol Plant Microbe In 2007; 20:659-70; PMID:17555274; http://dx.doi.org/10.1094/MPMI-20-6-0659
- Whitham SA, Quan S, Chang HS, Cooper B, Estes B, Zhu T, Wang X, Hou YM. Diverse RNA viruses elicit the expression of common sets of genes in susceptible Arabidopsis thaliana plants. Plant J 2003; 33:271-83; PMID:12535341; http://dx.doi.org/10.1046/j.1365-313X.2003.01625.x
- Huang Z, Yeakley JM, Garcia EW, Holdridge JD, Fan JB, Whitham SA. Salicylic acid-dependent expression of host genes in compatible Arabidopsis-virus interactions. Plant Physiol 2005; 137:1147-59; PMID:15728340; http://dx.doi.org/10.1104/pp.104.056028
- Lee WS, Fu SF, Li Z, Murphy AM, Dobson EA, Garland L, Chaluvadi SR, Lewsey MG, Nelson RS, Carr JP. Salicylic acid treatment and expression of an RNA-dependent RNA polymerase 1 transgene inhibit lethal symptoms and meristem invasion during tobacco mosaic virus infection in Nicotiana benthamiana. BMC Plant Biol 2016; 16:15; PMID:26757721; http://dx.doi.org/10.1186/s12870-016-0705-8
- Lewsey MG, Murphy AM, Maclean D, Dalchau N, Westwood JH, Macaulay K, Bennett MH, Moulin M, Hanke DE, Powell G, et al. Disruption of two defensive signaling pathways by a viral RNA silencing suppressor. Mol Plant-Microbe Interactions 2010; 23:835-45; PMID:20521947; http://dx.doi.org/10.1094/MPMI-23-7-0835
- Mayers CN, Lee KC, Moore CA, Wong SM, Carr JP. Salicylic acid-induced resistance to Cucumber mosaic virus in squash and Arabidopsis thaliana: contrasting mechanisms of induction and antiviral action. Mol Plant Microbe In 2005; 18:428-34; PMID:20521947; http://dx.doi.org/10.1094/MPMI-18-0428
- Wang SD, Zhu F, Yuan S, Yang H, Xu F, Shang J, Xu MY, Jia SD, Zhang ZW, Wang JH, et al. The roles of ascorbic acid and glutathione in symptom alleviation to SA-deficient plants infected with RNA viruses. Planta 2011; 234:171-81; PMID:21394469; http://dx.doi.org/10.1007/s00425-011-1391-2
- Mandadi KK, Scholthof KB. Plant immune responses against viruses: how does a virus cause disease? Plant Cell 2013; 25:1489-505; PMID:23709626; http://dx.doi.org/10.1105/tpc.113.111658
- Pagán I, Fraile A, Fernandez-Fueyo E, Montes N, Alonso-Blanco C, García-Arenal F. Arabidopsis thaliana as a model for the study of plant-virus co-evolution. Philos Trans R Soc B Biol Sci 2010; 365:1983-95; PMID:20478893; http://dx.doi.org/10.1098/rstb.2010.0062
- Roossinck MJ. Plant virus ecology. PLoS Pathog 2013; 9:e1003304; PMID:23717199; http://dx.doi.org/10.1371/journal.ppat.1003304
- Ryu CM, Murphy JF, Mysore KS, Kloepper JW. Plant growth-promoting rhizobacteria systemically protect Arabidopsis thaliana against Cucumber mosaic virus by a salicylic acid and NPR1-independent and jasmonic acid-dependent signaling pathway. Plant J 2004; 39:381-92; PMID:15255867; http://dx.doi.org/10.1111/j.1365-313X.2004.02142.x
- Epple P, Mack AA, Morris VR, Dangl JL. Antagonistic control of oxidative stress-induced cell death in Arabidopsis by two related, plant-specific zinc finger proteins. Proc Natl Acad Sci U S A 2003; 100:6831-6; PMID:12732715; http://dx.doi.org/10.1073/pnas.1130421100
- Carrère I, Tepfer M, Jacquemond M. Recombinants of cucumber mosaic virus (CMV): determinants of host range and symptomatology. Archives Virol 1999; 144:365-79; http://dx.doi.org/10.1007/s007050050510
- Gao R, Liu P, Wong S-M. Identification of a plant viral RNA genome in the nucleus. PloS One 2012; 7:e48736; PMID:23155403; http://dx.doi.org/10.1371/journal.pone.0048736
- Nguyen HD, Tomitaka Y, Ho SY, Duchene S, Vetten HJ, Lesemann D, Walsh JA, Gibbs AJ, Ohshima K. Turnip mosaic potyvirus probably first spread to Eurasian brassica crops from wild orchids about 1000 years ago. PloS One 2013; 8:e55336; PMID:23405136; http://dx.doi.org/10.1371/journal.pone.0055336
- Mason MG, Botella JR. Completing the heterotrimer: isolation and characterization of an Arabidopsis thaliana G protein g-subunit cDNA. Proc Natl Acad Sci U S A 2000; 97:14784-8; PMID:11121078; http://dx.doi.org/10.1073/pnas.97.26.14784
- Mason MG, Botella JR. Isolation of a novel G-protein g-subunit from Arabidopsis thaliana and its interaction with Gb. Biochim Biophys Acta 2001; 1520:147-53; PMID:11513956; http://dx.doi.org/10.1016/S0167-4781(01)00262-7
- Zvereva AS, Pooggin MM. Silencing and innate immunity in plant defense against viral and non-viral pathogens. Viruses 2012; 4:2578-97; PMID:23202495; http://dx.doi.org/10.3390/v4112578
- Gao M, Wang X, Wang D, Xu F, Ding X, Zhang Z, Bi D, Cheng YT, Chen S, Li X, et al. Regulation of cell death and innate immunity by two receptor-like kinases in arabidopsis. Cell Host Microbe 2009; 6:34-44; PMID:19616764; http://dx.doi.org/10.1016/j.chom.2009.05.019
- Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E, et al. A central role of salicylic acid in plant disease resistance. Science 1994; 266:1247-50; PMID:17810266; http://dx.doi.org/10.1126/science.266.5188.1247
- Hull R. Plant Virology. San Diego: Academic Press, 2002; http://dx.doi.org/10.1016/b978-012361160-4/50051-7
- Strawn MA, Marr SK, Inoue K, Inada N, Zubieta C, Wildermuth MC. Arabidopsis isochorismate synthase functional in pathogen-induced salicylate biosynthesis exhibits properties consistent with a role in diverse stress responses. J Biol Chem 2007; 282:5919-33; PMID:17190832; http://dx.doi.org/10.1074/jbc.M605193200
- Peng H, Li S, Wang L, Li Y, Li Y, Zhang C, et al. Turnip mosaic virus induces expression of the LRR II subfamily genes and regulates the salicylic acid signaling pathway in non-heading Chinese cabbage. Physiol Mol Plant P 2013; 82:64-72; http://dx.doi.org/10.1016/j.pmpp.2013.01.006
- Heo WD, Lee SH, Kim MC, Kim JC, Chung WS, Chun HJ, Lee KJ, Park CY, Park HC, Choi JY, et al. Involvement of specific calmodulin isoforms in salicylic acid-independent activation of plant disease resistance responses. Proc Natl Acad Sci USA 1999; 96:766-71; PMID:9892708; http://dx.doi.org/10.1073/pnas.96.2.766
- Sulistyowati E, Mitter N, Bastiaan-Net S, Roossinck MJ, Dietzgen RG. Host range, symptom expression and RNA 3 sequence analyses of six Australian strains of Cucumber mosaic virus. Australasian Plant Pathol 2004; 33:505-12; http://dx.doi.org/10.1071/AP04054
- German TL, Adkins S, Witherell A, Richmond KE, Knaack WR, Willis DK. Infection of Arabidopsis thaliana ecotype Columbia by Tomato spotted wilt virus. Plant Mol Biol Reporter 1995; 13:110-7; http://dx.doi.org/10.1007/BF02668780
- Purnell MP, Botella JR. Tobacco isoenzyme 1 of NAD(H)-dependent glutamate dehydrogenase catabolizes glutamate in vivo. Plant Physiol 2007; 143:530-9; PMID:17114271; http://dx.doi.org/10.1104/pp.106.091330
- Love AJ, Yun BW, Laval V, Loake GJ, Milner JJ. Cauliflower mosaic virus, a compatible pathogen of Arabidopsis, engages three distinct defense-signaling pathways and activates rapid systemic generation of reactive oxygen species. Plant Physiol 2005; 139:935-48; PMID:16169957; http://dx.doi.org/10.1104/pp.105.066803
- Lilly ST, Drummond RS, Pearson MN, MacDiarmid RM. Identification and validation of reference genes for normalization of transcripts from virus-infected Arabidopsis thaliana. Mol Plant Microbe In 2011; 24:294-304; PMID:21091160; http://dx.doi.org/10.1094/MPMI-10-10-0236
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Method 2001; 25:402-8; PMID:11846609; http://dx.doi.org/10.1006/meth.2001.1262
- Keogh RC, Deverall BJ, McLeod S. Comparison of histological and physiological responses to Phakopsora pachyrhizi in resistant and susceptible soybean. Transactions Br Mycological Society 1980; 74:329-33; http://dx.doi.org/10.1016/S0007-1536(80)80163-X
- Joo JH, Wang SY, Chen JG, Jones AM, Fedoroff NV. Different signaling and cell death roles of heterotrimeric G protein α and β subunits in the arabidopsis oxidative stress response to ozone. Plant Cell 2005; 17:957-70; PMID:15705948; http://dx.doi.org/10.1105/tpc.104.029603
