ABSTRACT
Cytokinin (CK) and glucose (GLC) control several common responses in plants. There is an extensive overlap between CK and GLC signal transduction pathways in Arabidopsis. Physiologically, both GLC and CK could regulate root length in light. CK interacts with GLC via HXK1 dependent pathway for root length control. Wild-type (WT) roots cannot elongate in the GLC free medium while CK-receptor mutant ARABIDOPSIS HISTIDINE KINASE4 (ahk4) and type B ARR triple mutant ARABIDOPSIS RESPONSE REGULATOR1, 10,11 (arr1, 10,11) roots could elongate even in the absence of GLC as compared with the WT. The root hair initiation was also found defective in CK signaling mutants ahk4, arr1,10,11 and arr3,4,5,6,8,9 on increasing GLC concentration (up to 3%); and lesser number of root hairs were visible even at 5% GLC as compared with the WT. Out of 941 BAP regulated genes, 103 (11%) genes were involved in root growth and development. Out of these 103 genes, 60 (58%) genes were also regulated by GLC. GLC could regulate 5736 genes, which include 327 (6%) genes involved in root growth and development. Out of these 327 genes, 60 (18%) genes were also regulated by BAP. Both GLC and CK signaling cannot alter root length in light in auxin signaling mutant AUXIN RESPONSE3/INDOLE-3-ACETIC ACID17 (axr3/iaa17) suggesting that they may involve auxin signaling component as a nodal point. Therefore CK- and GLC- signaling are involved in controlling different aspects of root growth and development such as root length, with auxin signaling components working as downstream target.
Introduction
The root system architecture mainly consists of primary root (PR), lateral roots (LR) and root hairs. In nature, root is constantly exposed to a variety of adverse environmental conditions such as temperature fluctuations, flood, drought, soil compaction, lack of essential nutrients, exposure to toxic minerals and pathogens, etc.Root is a highly dynamic structure and can adjust their growth as per changing environmental conditions by modulating its length; thickness; tropic movement; lateral root and root hair formation.Citation1,2,3,4,5,6 The optimal root architecture is essential to provide fitness to the plant in nature since it provides mechanical support to the aerial parts of the plant body, anchorage to the soil, helps in water and nutrients uptake from the soil, synthesize metabolites, can serve as energy storage organ, and interacts with symbiotic organisms.Citation3,4 Therefore, a detailed understanding of all the pathways and their cross-talk involved in root growth and development is an important prerequisite in plant biology.
Phytohormones play significant roles as a rapid endogenous signaling molecules to determine optimal root architecture in response to these cues.Citation7,8,9 The core of hormone regulatory network involved in shaping root growth pattern comprises of 2 well-known classical plant hormones, cytokinin (CK) and auxin, interacting at the levels of metabolism, transport, signaling and gene expression.Citation8,10,11,12,13,14 CKs are essential molecules playing key roles during different phases of plant growth and development.Citation15 CK signaling involves multistep phosphorelay which is the more advanced and complex version of bacterial 2 component system (TCS).Citation13,15,16 ARABIDOPSIS HISTIDINE KINASE2 (AHK2), AHK3 and AHK4 are membrane spanning proteins that serve as CK receptors and are primarily localized at the ER membrane.Citation17,18,19 Among CK receptors, AHK4 uniquely possesses dual activity since it leads to phosphorylation of AHPs in the presence of CK, while it causes dephosphorylation of AHPs in the absence of CK.Citation20 Five His phosphotransfer proteins (AHP1-AHP5) function as redundant positive regulators, whereas AHP6 (pseudo-HPt) serves as a negative regulator of CK signaling cascade.Citation21 AHPs continuously shuttle between the cytoplasm and nucleus independent of their phosphorylation status or of CK level. Only nuclear phosphorylated AHPs can phosphorylate ARABIDOPSIS RESPONSE REGULATORS/ARRs.Citation22 The ARRs are fall into 2 categories, type A ARRs (ARR3-ARR9, ARR15-ARR17) and type B ARRs (ARR1, ARR2, ARR10-ARR14, ARR18-ARR21). Phosphorylated type B response regulators can induce the transcription of type A ARRs, CYTOKININ RESPONSE FACTORS (CRFs) and some other CK early-responsive genes. Type A response regulators act as negative regulators and attenuates CK signaling whereas type B response regulators function as positive regulators of CK signaling cascade.Citation23
In literature, there are reports which suggest that sugar (Glucose/GLC) can also affect root growth and development.Citation3,24,25,26,27 GLC signaling pathways existing in Arabidopsis are mainly of 2 types; i) AtHXK1 (HEXOKINASE1) -dependent pathway (AtHXK1-signaling function dependent and AtHXK1-catalytic activity dependent) and, ii) AtHXK1-independent pathway which involves G-protein mediated signaling.Citation28 In AtHXK1 signaling function dependent pathway, HXK1 acts as an intracellular GLC sensor. HXK1 loss-of-function mutant (S177A mutation) glucose insensitive2.1 (gin2.1) retained the signaling function (GLC sensing) but not the catalytic activity (GLC phosphorylation).Citation29 In AtHXK1-independent pathway, plasma membrane bound protein regulator of G-protein signaling (RGS1) acts as an external glucose sensor.Citation30
Sugar and CK are key molecules in plants and there are several reports indicating that both these molecules regulate a myriad of similar processes. CK and GLC can act agonistically,Citation31,32,33,34 antagonistically,Citation29,35 and independent of each other.Citation36 Earlier our group reported a novel root directional response termed as “CK-induced root growth response” which is significantly enhanced when GLC is supplemented in the medium.Citation3 In addition, our group also reported that at whole genome level, large number of genes (89%) were agonistically regulated by GLC and CK.Citation37 GLC could also affect genes involved in CK biosynthesis, degradation and signaling.Citation37 Physiologically, both GLC and CK could control root growth in light, hypocotyl length in dark, chlorophyll and anthocyanin content.Citation37 Mishra et al. (2009) reported that increasing GLC concentrations affect primary root length, lateral root numbers, root hairs and gravitropic curvature of the roots of Arabidopsis seedlings.Citation25 Increasing GLC concentrations can also influence root length, lateral roots, root deviation and root hair elongation in auxin signaling mutants indicating that auxin signaling is involved in controlling GLC-regulated root phenotypes.Citation25 GLC acts through G-protein complex to attenuate auxin-mediated bimodality in regulating lateral root formation.Citation26 High GLC concentrations reduced root meristem size by repressing the accumulation of PIN1 and thus reducing auxin level.Citation27 In summary, CK, auxin and GLC control several root-related responses. Here, we are showing how CK- and GLC-signaling interact in controlling root growth and development in Arabidopsis.
Results
Involvement of GLC signaling components in controlling root growth
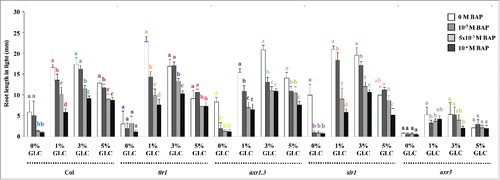
To find out the effect of CK and GLC on root length; light-grown 5-d-old Columbia (Col) seedlings were transferred to different concentrations of GLC and 6-benzylaminopurine (BAP) containing half strength MS medium in the light for 3-d as mentioned by Kushwah and Laxmi (2014).Citation37 BAP decreased the root length in Col at all the concentrations tested. GLC supplementation in the medium increased the root length. The increase in root length was found to be more at 1% GLC and 3% GLC, whereas found less at 5% GLC when compared with the untreated control ().Citation37 Thus for regulating primary root length, GLC and CK work antagonistically at lower GLC concentrations (1% and 3%) and act agonistically at higher GLC concentration (5%) (). CK-sensitivity of GLC signaling mutants was assayed to find out whether GLC-CK interaction controls root length via HXK1-dependent or HXK1-independent pathway. HXK1-dependent pathway mutant gin2 was found resistant/less sensitive at all the GLC concentrations tested () whereas HXK1-independent pathway mutants rgs1, G-PROTEIN ALPHA SUBUNIT1 (gpa1) and THYLAKOID FORMATION1 (thf1) were found to have similar sensitivity (), toward CK for root length control as compared with WT. These results altogether indicate that CK interacts with GLC via HXK1 dependent pathway to control root length.
Figure 1. GLC-CK signaling interaction in WT for root length control: BAP decreased the root length in Col at all the concentrations tested. GLC supplementation in the medium increased the root length. The increase in root length was found to be more at 1% GLC and 3% GLC, whereas found less at 5% GLC when compared with the untreated control. For controlling root length, GLC and CK work antagonistically at lower GLC concentrations but act agonistically at higher GLC concentration. Data shown is the average root length and error bars represent SD. Means accompanied by the different letters in a group at mentioned GLC concentration are statistically different (All Pairs, Tukey HSD post ANOVA test at P≤ 5%).
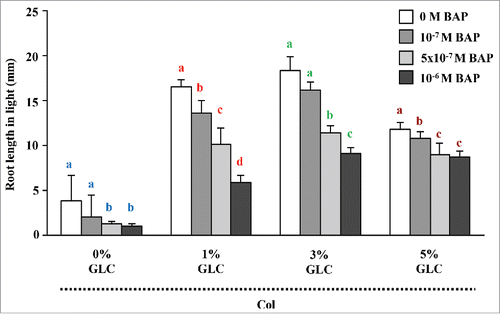
Figure 2. HXK1-dependence of CK signaling for root length control: Root growth of L. erecta and HXK1-dependent pathway mutant gin2.1. The gin2.1 mutant was found less sensitive at all the GLC concentrations tested to CK for root length control as compared with WT. Data shown is the average root length and error bars represent SD. Means accompanied by the different letters in a group at mentioned GLC concentration are statistically different (All Pairs, Tukey HSD post ANOVA test at P ≤ 5%).
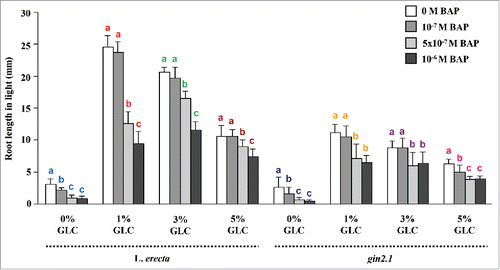
Figure 3. Root length of HXK1-independent pathway mutants: (A and B) The HXK1-independent pathway mutants rgs1.1, rgs1.2, thf1.1 and gpa1.1 were found to have similar sensitivity to CK for root length control as compared with WT. Data shown is the average root length and error bars represent SD. Means accompanied by the different letters in a group at mentioned GLC concentration are statistically different (All Pairs, Tukey HSD post ANOVA test at P ≤ 5%).
Involvement of CK signaling components in controlling root growth
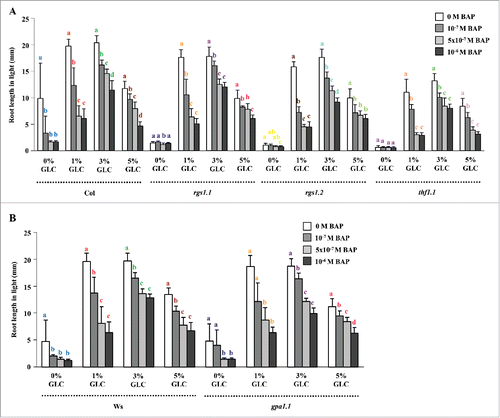
GLC-sensitivity of CK signaling mutants was assayed to find out the role of CK signaling elements in controlling root growth. In CK-perception mutant ahk4 and type B ARR mutant arr1,10,11; root length was found more even in the absence of GLC as compared with the WT ().
Figure 4. CK signaling dependence of GLC response for root length, lateral root numbers and root hairs: (A) In CK-receptor mutant ahk4 and type B ARR triple mutant arr1,10,11, root length was found to be more even in the absence of GLC as compared with the WT. (B) CK perception mutant ahk4, type B ARR triple mutant arr1,10,11 and type A ARR mutant arr3,4,5,6,8,9 displayed similar lateral root numbers as compared with the WT. (C) The root hair initiation was defective in ahk4, arr1,10,11 and arr3,4,5,6,8,9 on increasing GLC concentration and lesser number of root hairs were visible even on 5% GLC as compared with WT. Data shown is the average root length/lateral root numbers and error bars represent SD. Student's t test, P < 0.05, *control vs treatment.
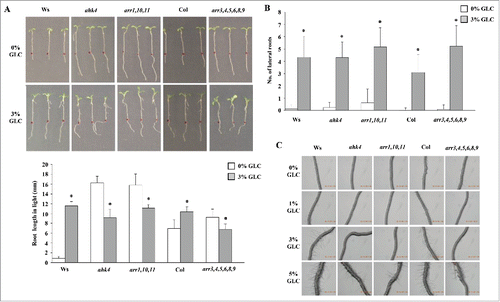
According to Mishra et al. (2009), GLC supplementation in the medium increases lateral roots in WT.Citation25 CK perception mutant ahk4, type B ARR triple mutant arr1,10,11 and type A ARR sextuple mutant arr3,4,5,6,8,9 displayed similar lateral root numbers as compared with the WT ().
The increasing GLC concentration also increases root hair initiation and elongation.Citation25 In auxin signaling mutants, GLC at higher concentrations could cause root hair initiation, however root hair elongation was severely compromised.Citation25 The root hair initiation was found defective in CK signaling mutants ahk4, arr1,10,11 and arr3,4,5,6,8,9 on increasing GLC concentration (up to 3%) and lesser number of root hairs were visible even at 5% GLC as compared with the WT ().
In microarray data published by Kushwah and Laxmi (2014), out of 941 genes regulated by BAP (0% GLC + 0 M BAP versus 0% GLC + 1 µM BAP), 103 (11%) genes were involved in root growth and development (Fig. S1A).Citation37 Out of these 103 genes, 60 (58%) genes were also regulated by GLC (Fig. S1A). Out of these 103 BAP-regulated genes which were involved in root growth and development, 6 genes were also involved in auxin biosynthesis and signaling (Fig. S1B). Out of these 6 genes, 4 genes were also regulated by GLC (Fig. S1B). Among the genes involved in auxin biosynthesis and signaling, one of the important member is SHORT HYPOCOTYL2 (SHY2)/ INDOLE-3-ACETIC ACID3 (IAA3) (Fig. S1B). The size of root meristem is established by a fine balance between the antagonistic interaction of auxin and CK which converges on SHY2.Citation38
GLC could regulate 5736 genes (0% GLC vs. 3% GLC), which include 327 (6%) genes involved in root growth and development (Fig. S2A). Out of these 327 genes, 60 (18%) genes were also regulated by BAP (Fig. S2A). Out of these 327 GLC-regulated genes which are involved in root growth and development, 12 genes are also involved in auxin biosynthesis and signaling (Fig. S2B). Out of these 12 genes, only one gene was also regulated by BAP (Fig. S2B). The genes involved in auxin biosynthesis and signaling are, LIKE AUXIN RESISTANT 1 (LAX1), ROOTY 1 (RTY1), PIN-FORMED 1 (PIN1), AUXIN-INDUCED IN ROOT CULTURES 12 (AIR12), AUXIN RESISTANT 4 (AXR4), HOMEOBOX 53 (HB53), P-GLYCOPROTEIN 1 (PGP1), MILDEW RESISTANCE LOCUS O 11 (MLO11), NAC DOMAIN CONTAINING PROTEIN 1 (NAC1), TRANSPARENT TESTA GLABRA 1 (TTG1) (Fig. S2B). These results altogether indicate that several genes involved in auxin signaling and homeostasis are also commonly regulated by CK and GLC.
Involvement of auxin signaling components in controlling root growth
CK and GLC sensitivity of auxin signaling mutants was assayed to find out if both these pathways culminate at auxin signaling or not. The auxin signaling mutants TRANSPORT INHIBITOR RESPONSE1 (tir1), AUXIN RESISTANT1.3 (axr1.3) and Aux/IAA gain of function mutant SOLITARY ROOT1 (slr1)/ INDOLE-3-ACETIC ACID14 (iaa14) were found to have similar sensitivity, whereas Aux/IAA gain of function mutant AUXIN RESPONSE3 (axr3)/INDOLE-3-ACETIC ACID17 (iaa17) was found highly resistant to different concentrations of CK and GLC for root length control as compared with the WT ().
Figure 5. Involvement of auxin signaling downstream to both GLC and CK in controlling root growth: The auxin signaling mutants tir1, axr1.3 and slr1 were found to have similar sensitivity whereas, axr3 mutant was found highly resistant for different concentrations of GLC and CK for root length control as compared with the WT. Data shown is the average root length and error bars represent SD. Means accompanied by the different letters in a group at mentioned GLC concentration are statistically different (All Pairs, Tukey HSD post ANOVA test at P≤ 5%).
Discussion
There are various reports in literature which indicates that for many plant responses, GLC and CK act antagonistically whereas, for others they act agonistically and for some responses both these molecules function independently of each other.Citation3,29,31,32,33,34,35,36,37
HXK-dependent pathway mutant gin2 was found less sensitive to all the GLC concentrations tested toward CK in terms of root length inhibition, whereas HXK-independent pathway mutants rgs1, gpa1 and thf1 were found to have similar sensitivity toward CK for root length regulation as compared with the WT. Therefore, similar to hypocotyl length regulation in dark, for root length control also CK interacts with GLC via AtHXK1-dependent pathway.Citation37
In CK-perception mutant ahk4 and type B ARR triple mutant arr1,10,11, root length was found to be more even in the absence of GLC as compared with the WT. This suggests that the positive elements of CK signaling might contribute toward non-elongation of root in the absence of GLC. The root length changes were significantly compromised in several auxin signaling mutants on increasing GLC concentration.Citation25 This suggests that CK and GLC signaling may also work via affecting auxin signaling as a downstream component.
CK perception mutant ahk4, type B ARR triple mutant arr1,10,11 and type A ARR sextuple mutant arr3,4,5,6,8,9 displayed similar lateral root numbers as compared with the WT. Lateral root induction was significantly compromised in auxin perception mutant tir1 and signaling mutants slr1/iaa14, axr2/iaa7 and axr3/iaa17 on increasing GLC concentration.Citation25 This suggests that GLC mediated regulation of lateral root numbers involve auxin signaling rather than CK signaling.
The root hair initiation was found defective in CK signaling mutants ahk4, arr1,10,11 and arr3,4,5,6,8,9 and only few root hairs were visible at high GLC concentration (5%). The root hair density and length significantly affect the root-soil interface.Citation6 According to Mishra et al. (2009), increasing GLC concentrations promote root hair initiation but the root hair elongation is compromised in auxin mutants indicating significant role of auxin signaling in GLC mediated root hair elongation.Citation25 High GLC is able to overcome the repression of root hair initiation in AUX/IAA gain-of-function mutants (root hairless mutants) slr1, axr2 and axr3.Citation25 This suggests that for GLC mediated root hair initiation CK signaling is involved whereas, for GLC mediated root hair elongation auxin signaling is involved.
Altogether, these results suggest that CK receptor, type B and type A RRs are involved in various GLC induced root growth and developmental changes. There are previous reports showing that type A ARRs integrate CK signaling with circadian rhythm, light, pathogen and other hormone signaling cascades.Citation38 The phosphorylated ARR4 interacts with phyB and therefore serves as a converging node for the integration of CK and light signaling.Citation39,40 For shoot meristem development and root stem-cell niche establishment, auxin and CK signaling converges on ARR7 and ARR15 (type A RRs).Citation41 Type A ARRs are involved in GLC mediated control of hypocotyl length in dark.Citation37 Here, we are reporting that for GLC-CK interaction, CK receptor as well as type A and type B RRs act as downstream components.
It is now evident from several studies that physiologic phenomenon are regulated in a complex web like manner by the interaction of several hormones.Citation42 CK is known to interact either in synergistic or antagonistic manner with other hormones, especially ethylene and auxin.Citation38,43,44 There are several reports indicating the cross-talk between auxin and CK in controlling root and lateral roots growth and development.Citation14,15,38,41,45,46 There are also various reports confirming that interaction between sugar and auxin controls root and lateral roots growth and development.Citation25,26,47 According to Mishra et al. (2009), increasing GLC concentrations can affect primary root length, gravitropism, lateral roots and root hairs in auxin perception and signaling mutants (TIR1, AXR2/IAA7, AXR3/IAA17 and SLR1/IAA14).Citation25 GLC acts via G-protein signaling to attenuate the auxin-mediated bimodality during lateral root formation.Citation26 In Arabidopsis, N-MYC DOWNREGULATED-LIKE1 (NDL1) protein interacts with Gβγ dimers, establish local auxin maxima and thus regulate lateral root initiation and emergence.Citation47 ABA INSENSITIVE 5 (ABI5) by repressing PIN1 accumulation functions in the GLC-mediated inhibition of the root meristem zone.Citation27 These reports in literature suggest that GLC modulates root growth via affecting auxin signaling and/or transport. The resistance of AUX/IAA gain-of-function mutant axr3 toward GLC and CK for root length control suggests that both these pathways may converge at AXR3 protein for root length regulation. Therefore, auxin signaling might act as a nodal point for GLC-CK interaction in controlling root length. There are also reports about the functional importance of cross-talk between auxin, CK and GLC signaling in regulating various developmental processes.Citation3,37 Resistance of axr3 toward GLC and CK for hypocotyl length control also suggests that GLC and CK signaling may converge at AUX/IAA proteins for controlling hypocotyl length in dark.Citation37 All these results together indicate that in addition to hypocotyl growth, CK- and GLC- signaling are also involved in controlling different aspects of root growth and development such as primary root length and root hairs with auxin signaling components as a downstream target. Understanding of key genetic components involved in the determination of optimal root system architecture can help us to engineer better performing crops under adverse environmental conditions.
Materials and methods
Plant materials
Following seed stocks were obtained from Arabidopsis Biological Resource Center (ABRC): ahk4 (CS6563, At2g01830); arr1,10,11 (CS6993, At3g16857/At4g31920/At1g67710); arr3,4,5,6,8,9 (CS25279, At1g59940/At1g10470/At3g48100/At5g62920/At2g41310/At3g57040); tir1.1 (CS3798, At3g62980); axr1.3 (CS3075, At1g05180); axr3.1 (CS57504, At1g04250); gin2.1 (CS6383, At4g29130). The rgs1.1 and rgs1.2 (At3g26090);Citation48 gpa1.1 (At2g26300);Citation49 thf1.1 (At2g20890)Citation50 lines were procured from the original published source. The slr1 (At4g14550) mutant line was provided by Dr. Hidehiro Fukaki (Nara Institute of Science and Technology, Nara, Japan).Citation51 The ahk4; arr1,10,11; gpa1.1 were in the Ws background, gin2.1 mutant was derived from Ler background whereas all other mutant lines used were in the Col background. All chemicals were purchased from Sigma (St. Louis, MO, USA). The BAP stock was prepared in DMSO (dimethyl sulfoxide).
Seedling growth
Seeds were sterilized, imbibed at 4˚C for 2-d and were sown on plates (120 × 120 mm) containing half-strength MS medium (pH 5.7) supplemented with 1% sucrose and 0.8% agar. Seed germination was performed in growth room under long day conditions (16 h light/8 h dark, 80 µmol m−2 s−1 light intensity and 22˚C ± 2˚C temperature). Afterwards, 5-d-old seedlings were transferred on plates (120 × 120 mm) containing half-strength MS medium (pH 5.7) supplemented with GLC (0%, 1%, 3%, 5%), BAP (0M, 10−7M, 5 × 10−7M, 10−6M) and agar (0.8%) and root tips were marked. Thereafter, seedlings were grown vertically for next 3-d in climate controlled long day growth chambers.
Measurement of root length and lateral roots
For root length, digital images were captured using Nikon Coolpix digital camera on the 3rd-d of seedling transfer and lengths were quantified using ImageJ. For root hairs digital images were captured with Nikon Coolpix digital camera connected with a Nikon SMZ1500 Stereo-Zoom microscope. Lateral roots were quantified directly under Nikon SMZ1500 Stereo-Zoom microscope on 3rd-d of seedling transfer. Data shown is the average of 10–15 seedlings and error bars represent SD. Student's t test with paired 2-tailed distribution and all pairs Tukey HSD post ANOVA test at P ≤ 5% were used as mentioned specifically for statistical analysis.
Disclosure of potential conflicts of interest
No potential conflict of interest were disclosed.
Supplemental_Material.zip
Download Zip (44.4 KB)Funding
This work was financially supported by the National Institute of Plant Genome Research (NIPGR) core grant, Department of Biotechnology, Government of India (Grant No. BT/PR14398/BRB/843/2010) and Department of Biotechnology, Government of India research fellowship to SK.
References
- Massa GD, Gilroy S. Touch modulates gravity sensing to regulate the growth of primary roots of Arabidopsis thaliana. Plant J 2003a; 33:435-45; PMID:12581302; https://doi.org/10.1046/j.1365-313X.2003.01637.x
- Massa GD, Gilroy S. Touch and gravitropic set-point angle interact to modulate gravitropic growth in roots. Adv Space Res 2003b; 31:2195-202; PMID:14686432; https://doi.org/10.1016/S0273-1177(03)00244-8
- Kushwah S, Jones AM, Laxmi A. Cytokinin interplay with ethylene, auxin, and glucose signaling controls Arabidopsis seedling root directional growth. Plant Physiol 2011; 156:1851-66; PMID:21666052; https://doi.org/10.1104/pp.111.175794
- Cuesta C, Wabnik K, Benková E. Systems approaches to study root architecture dynamics. Systems approaches to study root architecture dynamics. Front Plant Sci 2013; 4:537; PMID:24421783; https://doi.org/10.3389/fpls.2013.00537
- Koevoets IT, Venema JH, Elzenga JTM, Testerink C. Roots withstanding their environment: Exploiting root system architecture responses to abiotic stress to improve crop tolerance. Front Plant Sci 2016; 7:1335; PMID:27630659; https://doi.org/10.3389/fpls.2016.01335
- Slovak R, Ogura T, Santosh BS, Ristova D, Busch W. Genetic control of root growth: From genes to networks. Ann Bot 2016; 117:9-24; PMID:26558398; https://doi.org/10.1093/aob/mcv160
- Malamy JE. Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ 2005; 28:67-77; PMID:16021787; https://doi.org/10.1111/j.1365-3040.2005.01306.x
- Den Herder G, Van Isterdael G, Beeckman T, De Smet I. The roots of a new green revolution. Trends Plant Sci 2010; 15:600-7; PMID:20851036; https://doi.org/10.1016/j.tplants.2010.08.009
- Wachsman G, Sparks EE, Benfey PN. Genes and networks regulating root anatomy and architecture. New Phytol 2015; 208(1):26-38; PMID:25989832; https://doi.org/10.1111/nph.13469
- Buer CS, Muday GK. The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell 2004; 16:1191-205; PMID:15100399; https://doi.org/10.1105/tpc.020313
- Pernisová M, Kuderová A, Hejátko J. Cytokinin and auxin interactions in plant development: Metabolism, signalling, transport and gene expression. Curr Protein Pept Sci 2011; 12:137-47; PMID:21348844; https://doi.org/10.2174/138920311795684887
- Vanstraelen M, Benková E. Hormonal interactions in the regulation of plant development. Annu Rev Cell Dev Biol 2012; 28:463-87; PMID:22856461; https://doi.org/10.1146/annurev-cellbio-101011-155741
- El-Showk S, Ruonala R, Helariutta Y. Crossing paths: Cytokinin signalling and crosstalk. Dev 2013; 140:1373-83; PMID:23482484; https://doi.org/10.1242/dev.086371
- Schaller GE, Bishopp A, Kieber JJ. The Yin-Yang of hormones: Cytokinin and auxin interactions in plant development. Plant Cell 2015; 27:44-63; PMID:25604447; https://doi.org/10.1105/tpc.114.133595
- Kieber JJ, Schaller GE. Cytokinins. Arabidopsis Book 2014; 12:e0168; PMID:24465173; https://doi.org/10.1199/tab.0168
- Pekárová B, Szmitkowska A, Dopitová R, Degtjarik O, Žídek L, Hejátko J. Structural aspects of multistep phosphorelay mediated signaling in plants. Mol Plant 2016; 9:71-85; PMID:26633861; https://doi.org/10.1016/j.molp.2015.11.008
- Caesar K, Thamm AM, Witthoft J, Elgass K, Huppenberger P, Grefen C, Horak J, Harter K. Evidence for the localization of the Arabidopsis cytokinin receptors AHK3 and AHK4 in the endoplasmic reticulum. J Exp Bot 2011; 62:5571-80; PMID:21841169; https://doi.org/10.1093/jxb/err238
- Lomin SN, Yonekura-Sakakibara K, Romanov GA, Sakakibara H. Ligand-binding properties and subcellular localization of maize cytokinin receptors. J Exp Bot 2011; 62:5149-59; PMID:21778179; https://doi.org/10.1093/jxb/err220
- Wulfetange K, Lomin SN, Romanov GA, Stolz A, Heyl A, Schmulling T. The cytokinin receptors of Arabidopsis thaliana are locating mainly to the endoplasmic reticulum. Plant Physiol 2011; 156:1808-18; PMID:21709172; https://doi.org/10.1104/pp.111.180539
- Mahonen AP, Higuchi M, Tormakangas K, Miyawaki K, Pischke MS, Sussman MR, Helariutta Y, Kakimoto T. Cytokinins regulate a bidirectional phosphorelay network in Arabidopsis. Curr Biol 2006; 16:1116-22; PMID:16753566; https://doi.org/10.1016/j.cub.2006.04.030
- Hutchison CE, Kieber JJ. Signaling via histidinecontaining phosphotransfer proteins in Arabidopsis. Plant Signal Behav 2007; 2:287-9; PMID:19704684; https://doi.org/10.4161/psb.2.4.4039
- Punwani JA, Hutchison CE, Schaller GE, Kieber JJ. The subcellular distribution of the Arabidopsis histidine phosphotransfer proteins is independent of cytokinin signaling. Plant J 2010; 62:473-82; PMID:20136728; https://doi.org/10.1111/j.1365-313X.2010.04165.x
- Hwang I, Sheen J. Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature 2001; 413:383-9; PMID:11574878; https://doi.org/10.1038/35096500
- Rolland F, Baena-Gonzalez E, Sheen J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu Rev Plant Biol 2006; 57:675-709; PMID:16669778; https://doi.org/10.1146/annurev.arplant.57.032905.105441
- Mishra BS, Singh M, Aggrawal P, Laxmi A. Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. PLoS One 2009; 4:e4502; PMID:19223973; https://doi.org/10.1371/journal.pone.0004502
- Booker KS, Schwarz J, Garrett MB, Jones AM. Glucose attenuation of auxin-mediated bimodality in lateral root formation is partly coupled by the heterotrimeric G protein complex. PLoS One 2010; 5:e12833; PMID:20862254; https://doi.org/10.1371/journal.pone.0012833
- Yuan TT, Xu HH, Zhang KX, Guo TT, Lu YT. Glucose inhibits root meristem growth via ABA INSENSITIVE 5, which represses PIN1 accumulation and auxin activity in Arabidopsis. Plant Cell Environ 2014; 37(6):1338-50.
- Xiao W, Sheen J, Jang JC. The role of hexokinase in plant sugar signal transduction and growth and development. Plant Mol Biol 2000; 44:451-61; PMID:11197321; https://doi.org/10.1023/A:1026501430422
- Moore B, Zhou L, Rolland F, Hall Q, Cheng WH, Liu YX, Hwang I, Jones T, Sheen J. Role of the Arabidopsis glucose sensor HXK1 in nutrient, light and hormonal signaling. Science 2003; 300:332-6; PMID:12690200; https://doi.org/10.1126/science.1080585
- Urano D, Jones AM. Heterotrimeric G protein-coupled signaling in plants. Ann Rev Plant Biol 2014; 65:365-84; PMID:24313842; https://doi.org/10.1146/annurev-arplant-050213-040133
- Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JAH. Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 1999; 283:1541-4; PMID:10066178; https://doi.org/10.1126/science.283.5407.1541
- Riou-Khamlichi C, Menges M, Healy JMS, Murray JAH. Sugar control of the plant cell cycle: Differential regulation of Arabidopsis D-type cyclin gene expression. Mol Cell Biol 2000; 20:4513-21; PMID:10848578; https://doi.org/10.1128/MCB.20.13.4513-4521.2000
- Hartig K, Beck E. Crosstalk between auxin, cytokinins, and sugars in the plant cell cycle. Plant Biol (Stuttg) 2006; 8:389-96; PMID:16807832; https://doi.org/10.1055/s-2006-923797
- Das PK, Shin DH, Choi S-B, Yoo S-D, Choi G, Park Y-I. Cytokinins enhance sugar-induced anthocyanin biosynthesis in Arabidopsis. Mol Cells 2012; 34:93-101; PMID:22699753; https://doi.org/10.1007/s10059-012-0114-2
- Franco-Zorrilla JM, Martin AC, Leyva A, Paz-Ares J. Interaction between phosphate-starvation, sugar, and cytokinin signaling in Arabidopsis and roles of cytokinin receptors CRE1/AHK4 and AHK2. Plant Physiol 2005; 138:847-57; PMID:15923327; https://doi.org/10.1104/pp.105.060517
- Aki T, Konishi M, Kikuchi T, Fujimori T, Yoneyama T, Yanagisawa S. Distinct modulations of the hexokinase1-mediated glucose response and hexokinase1-independent processes by HYS1/CPR5 in Arabidopsis. J Exp Bot 2007; 58:3239-48; PMID:17720689; https://doi.org/10.1093/jxb/erm169
- Kushwah S, Laxmi A. The interaction between glucose and cytokinin signal transduction pathway in Arabidopsis thaliana. Plant Cell Environ 2014; 37:235-53; PMID:23763631; https://doi.org/10.1111/pce.12149
- Hwang I, Sheen J, Müller B. Cytokinin signaling networks. Annu Rev Plant Biol 2012; 63:353-80; PMID:22554243; https://doi.org/10.1146/annurev-arplant-042811-105503
- Sweere U, Eichenberg K, Lohrmann J, Mira-Rodado V, Baurle I, Kudla J, Nagy F, Schafer E, Harter K. Interaction of the response regulator ARR4 with phytochrome B in modulating red light signaling. Science 2001; 294:1108-11; PMID:11691995; https://doi.org/10.1126/science.1065022
- Mira-Rodado V, Sweere U, Grefen C, Kunkel T, Fejes E, Nagy F, Schäfer E, Harter K. Functional cross-talk between two component and phytochrome B signal transduction in Arabidopsis. J Exp Bot 2007; 58:2595-607; PMID:17545225; https://doi.org/10.1093/jxb/erm087
- Müller B, Sheen J. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 2008; 453:1094-7; PMID:18463635; https://doi.org/10.1038/nature06943
- Munné-Bosch S, Müller M. Hormonal cross-talk in plant development and stress responses. Front Plant Sci 2013; 4:529; PMID:24400014; https://doi.org/10.3389/fpls.2013.00529
- Rashotte AM, Chae HS, Maxwell BB, Kieber JJ. The interaction of cytokinin with other signals. Physiol Plant 2005; 123:184-94; https://doi.org/10.1111/j.1399-3054.2005.00445.x
- Su YH, Liu YB, Zhan XS. Auxin-cytokinin interaction regulates meristem development. Mol Plant 2011; 4:616-25; PMID:21357646; https://doi.org/10.1093/mp/ssr007
- Dello Ioio R, Nakamura K, Moubayidin L, Perilli S, Taniguchi M, Morita MT, Aoyama T, Costantino P, Sabatini S. A genetic framework for the control of cell division and differentiation in the root meristem. Science 2008; 322:1380-4; PMID:19039136; https://doi.org/10.1126/science.1164147
- Laplaze L, Benkova E, Casimiro I, Maes L, Vanneste S, Swarup R, Weijers D, Calvo V, Parizot B, Herrera-Rodriguez MB, et al. Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell 2007; 19:3889-900; PMID:18065686; https://doi.org/10.1105/tpc.107.055863
- Mudgil Y, Uhrig JF, Zhou J, Temple B, Jiang K, Jones AM. Arabidopsis N-MYC DOWNREGULATED-LIKE1, a positive regulator of auxin transport in a G protein-mediated pathway. Plant Cell 2009; 21:3591-609; PMID:19948787; https://doi.org/10.1105/tpc.109.065557
- Chen J-G, Willard FS, Huang J, Liang J, Chasse SA, Jones AM, Siderovsky DP. A seven-transmembrane RGS protein that modulates plant cell proliferation. Science 2003; 301:1728-31; PMID:14500984; https://doi.org/10.1126/science.1087790
- Ullah H, Chen J-G, Young JC, Im K-H, Sussman MR, Jones AM. Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science 2001; 292:2066-9; PMID:11408654; https://doi.org/10.1126/science.1059040
- Huang J, Taylor JP, Chen J-G, Uhrig JF, Schnell DJ, Nakagawa T, Korth KL, Jones AM. The plastid protein THYLAKOID FORMATION1 and the plasma membrane G-protein GPA1 interact in a novel sugar signaling mechanism in Arabidopsis. Plant Cell 2006; 18:1226-38; PMID:16582010; https://doi.org/10.1105/tpc.105.037259
- Fukaki H, Tameda S, Masuda H, Tasaka M. Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J 2002; 29:153-68; PMID:11862947; https://doi.org/10.1046/j.0960-7412.2001.01201.x
