ABSTRACT
Experimental work has shown that Boron (i.e., Boric acid, B) is an essential and multifunctional microelement for vascular plant development. In addition to its other functions, which include xylem development and lignin biosynthesis, we now know that B is involved in phytohormone-signaling and influences the mechanical properties of intercellular pectins. From these data, we conclude that B played an important role during the evolutionary development of lignified tissues, and that it may have been involved in the evolution of vascular plant roots, as hypothesized by D. H. Lewis in 1980. Herein, we review the data pertaining to Lewis’ hypothesis, present experimental results on the role of B in root (vs. rhizoid) formation in sunflower vs. a liverwort, and describe the appearance of roots in the fossil record. Open questions are addressed, notably the lack of our knowledge concerning soil microbes and their interactive roles with the micronutrient B during root formation.
Introduction
Thirty-seven years ago, LewisCitation1 hypothesized that the micronutrient Boron (i.e., Boric acid, B) played an essential role during the evolutionary transition from non-vascular (bryophyte-like) plants to vascularized embryophytes during the early Paleozoic. Specifically, LewisCitation1 summarized evidence indicating that B was required for the biosynthesis of lignin. Moreover, in conjunction with the phytohormone auxin, B may induce the differentiation of xylem-elements. Since B was found to be necessary for the development of adventitious roots, LewisCitation1 postulated that this micronutrient may have been a key factor for the origin of the root system in the earliest vascular plants.
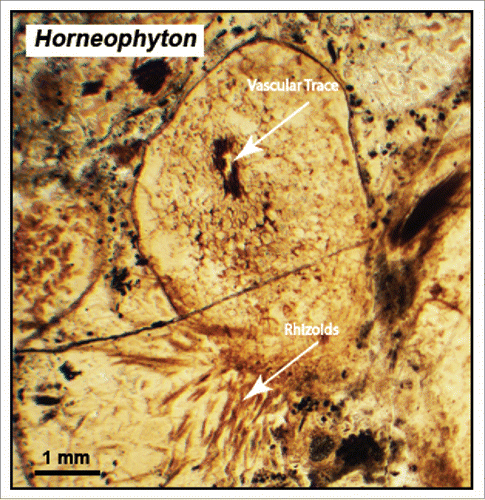
In a Review Article on “Early Root Evolution," Kenrick and Strullu-DerrienCitation2 provide paleontological data documenting the occurrence of rhizoids in bryophyte-like Rhynie Chert fossils, such as the ca. 407 million-year (Myr)-old Horneophyton (), and roots on sporophytes of some early vascular plants, such as Zosterophyllum of about the same age. In 2016, Gerrienne et al.Citation3 summarized this topic from a paleontological perspective. With respect to the physiologic basis of rhizoid/root formation in the most ancient embryophytes, Kenrick and Strullu-DerrienCitation2 discuss the possible role of polar auxin transport. However, in both articles, the authorsCitation2,3 do not mention the hypothesis of Lewis,Citation1 nor some of the other important functions Boron performs in developing land plants. These topics, notably the effects of this micronutrient (as well as epiphytic microbes) on root development in seed plants,Citation4,5 such as Arabidopsis thaliana or Helianthus annuus, are the focus of the present article.
Evolutionary gains and losses of roots
Roots and root-like organs have evolved independently many times among the vascular plants (i.e., twice in the lycopods and perhaps as many as 3 times in the euphyllophytes).Citation6,7 The capacity to form roots has also been lost or repressed among some extant tracheophytes, as for example among some eusporangiate “ferns” (e.g., Psilotum nudum) and some angiosperms (e.g., Wolffia arrhiza).Citation8 The evolutionary origins of roots are unfortunately ambiguous because it is often difficult to determine if roots are absent, as in some extant pteridophytes, not preserved for a particular fossil, or perhaps may not be recognized in a fossilized state. Another factor contributing to the problematic nature of root evolution is the paucity of definitive developmental features that can be used to define accurately and precisely what is meant by “roots." This ambiguity is attributable in part to the fact that roots have evolved many times, and therefore in different ways among tracheophytes. For example, root development is typically described as endogenous, i.e., the apical meristems of lateral roots develop from cells within the pericycle. However, the roots of some ferns (e.g., Ceratopteris) emerge from cells in the outermost layer of the cortex just below the epidermis (whose cells divide anticlinally to keep pace with divisions in the hypodermis) in a manner that is essentially indistinguishable from the ontogeny of eudicot leaves,Citation9,10 wherein the initial bulge of the leaf primordium forms as a result of sub-epidermal and epidermal cell divisions. In passing, it is worth noting that the sub-epidermal origin of the roots of Ceratopteris and some other leptosporangiate ferns is virtually indistinguishable from that of stigmarian roots, which are considered by many workers to be leaf homologs.Citation10 Likewise, the application of other criteria typically used to distinguish roots from other organ types is equally problematic. For example, an endodermis with a Casparian strip occurs in the stems of some lycopods (e.g., Hupertzia) and the leaves of some conifers (e.g., Pinus), and the aerial roots of some grasses lack or possess poorly developed rootcaps (which would have a low probability of being preserved as fossils in some geological contexts).
Finally, it should not escape notice that even among the monophyletic angiosperms, the root apical meristem has at least 15 demonstrably different anatomic configurations.Citation10 Consequently, the application of the concept of endogenous development or any other developmental or anatomic criteria to define “roots” can be misleading. It should also be noted that, based on a single model organism such as Arabidopsis thaliana, the multiple evolutionary origins of organs called “roots” may preclude drawing undisputed conclusions about their evolution.
Root evolution and the fossil record
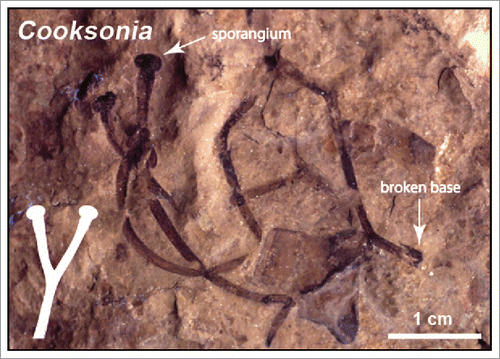
If we visualize the biology of the first terrestrial embryophytes () based on the biology of extant non-vascular embryophytes (i.e., liverworts, mosses, and hornworts), the first plants to actually live on the land (literally) were gametophytes and not their associated sporophytes.Citation11,12 This postulate emerges from the fact that the sporophytes of liverworts, mosses, and hornworts invariably begin their development within archegonia and gain water and other nutrients by a basipetal “foot” (via matrotrophy) during their early ontogeny. This “model” for the dependency of the first embryophyte sporophytes on their gametophytes is consistent with (albeit not substantiated by) the megafossil remains of some of the earliest known vascular plant sporophytes, e.g., Cooksonia (). With the exception of one report purporting to show Cooksonia sporophytes attached at their base to a gametophyte,Citation3 the proximal portions of all other Cooksonia sporophytes are broken and provide no evidence for what the morphology of the basal portions of the sporophyte or the gametophyte looked like. In passing, it is worth noting that the genus “Cooksonia” should be considered a form genus and not a “true genus." The morphological diversity of the sporangia on structures described as Cooksonia is so great that it is unlikely that all morphs belong to the same organism. It is nevertheless certain that many of the first vascular plants lacked roots owing to the exquisite preservation of the plants preserved in the Rhynie Chert (e.g., Rhynia and Horneophyton). These early land plants absorbed water and nutrients by means of rhizoids and their association with mycorrhizae.
Figure 2. Morphology of the sporophyte of the Lower Devonian land plant Zosterophyllum shengfengense, which may have inhabited moist (semi-aquatic) areas. Note that the basal part of the plant is depicted as consisting of numerous negatively gravitropic root-like organs. Adapted from Hao et al..Citation13
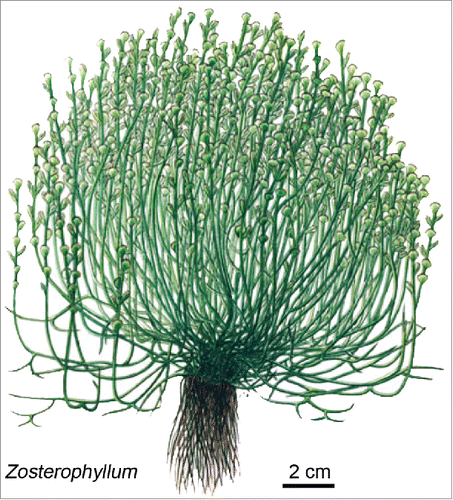
Figure 3. Compression fossil assigned to the early Paleozoic genus Cooksonia and schematic of a stereotypical sporophyte (see Inset). (Specimen from the Cornell University fossil plant collection).
Finally, the oldest known fossil plant with roots, Zosterophyllum shengfengense from Southern China,Citation14 predates the Rhynie chert and lacked leaves (see ). Thus, until future discoveries in the fossil record indicate otherwise, the evolution of roots preceded the phylogenetic development of the organographic distinction between what is conventionally meant by “leaf” and “stem."
Boron as a multifunctional microelement
Seven years ago, we summarized paleobotanical as well as developmental and molecular data indicating that embryophytes originated from a common ancestor similar to extant freshwater charophycean algae.Citation11,12 In that context, we discussed the morphology of mature sporophytes of Zosterophyllum rhenatum, an Early Devonian (ca. 410 Myr-old) tracheophyte – the root-less specimen depicted in our article was collected in the Ahrtal/Rhineland, Germany. Concomitantly, Hao et al.Citation13 described a newly discovered, uprooted specimen of Zosterophyllum from Southern China, Z. shengfengense. This ca. 413 Myr-old land plant has a large rhizome-like rooting system, and is considered to be the oldest known tracheophyte.Citation2 The re-constructed phenotype of Z. shengfengense () depicts positively gravitropic axes attached to the shoot, which are reminiscent of adventitious roots in the majority of extant tracheophytes.
What is the significance of Lewis’ “Boron-hypothesis” with respect to the evolutionary appearance of roots? Before answering this question, it must be stated that the precise metabolic function of B (translocated via aquaporins) in plant development is unclear.Citation5 Empirical observations, however, indicate that it is involved in cell expansion, nucleic acid synthesis, membrane function, hormone responses, pollen tube growth, and lignin biosynthesis (see, Citationrefs 14–18). For this reason, B-deficiency exhibits a variety of symptoms including the necrosis of leaf lamina and terminal buds, the suppression of apical dominance and cell expansion, and the breakdown of parenchyma in fleshy roots, tubers, and fruits. In Arabidopsis, B-limitation results in an inhibition of root elongation, accompanied by a boost in root hair elongation (which can also be induced by methylotrophic bacteria).Citation4,5
These phenomena are explicable in part because Boron plays an important role in embryophyte cell-to-cell adhesion. Specifically, B forms a covalent complex with Ca2+–rhamnogalacturonic II (RG-II), and the structure of the B-RG-II complex (which consists of 2 molcules of Boron, Ca2+, and 2 chains of monomeric RG-II) is ubiquitous in the sporophyte and gametophyte generations of vascular land plants.Citation16
Root formation vs. the occurrence of rhizoids
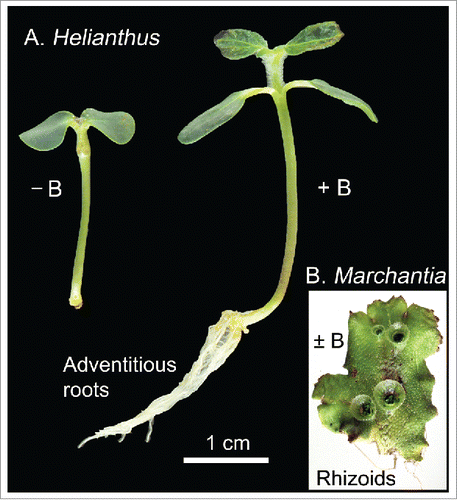
In addition to the numerous roles B plays, LewisCitation1 drew attention to the classic study of Middleton et al.,Citation17 wherein it is shown that the growth of adventitious roots in bean (Phaseolus aureus)-cuttings is promoted by B. More recent studies verified and extended this observation. Josten and KutscheraCitation18 documented that when light-grown seedlings of sunflower (Helianthus annuus) are de-rooted and incubated on nutrient solution containing or lacking boric acid ( ± B), adventitious roots developed in the + B-samples (up to 10 per cutting), but not in the – B-samples. This B-induced development of adventitious roots occurred in the absence of any externally applied phytohormones (e.g., auxins, gibberellins, cytokinins). Cytological studies revealed that B stimulated the meristematic activity of the internal cells of the hypocotyl, and hence initiated the development of juvenile roots at the cut end.Citation18
shows that 4-d-old light-grown seedlings, de-rooted and placed in an upright position 5 mm deep into moist vermiculite (nutrient solution ± B), develop a large system of adventitious roots in the presence of the micronutrient. The cuttings with adventitious roots were planted in soil and incubated in a light/day-growth chamber. All of these seedlings survived and flowered. Under similar experimental conditions, pieces of the liverwort Marchiantia polymorpha, placed on agar with a nutrient solution ( ± B), as described by Kutschera and Koopmann,Citation19 developed numerous rhizoids, both in the presence and absence of the micronutrient (). These results show that adventitious roots are induced by B, whereas rhizoid development is independent of this micronutrient. This finding is consistent with the facts that borate-cross-linked pectic polysaccharides are present in the cell walls of sporophytes of vascular plants (sunflower etc.), but largely absent in the walls of the gametophytes of the nonvascular bryophytes (liverworts, mosses, hornworts).Citation20
Figure 4. Effect of boric acid (B) on the development of adventitious roots in cuttings from light-grown sunflower (Helianthus annuus) plants. Four-day-old seedlings were de-rooted, placed with the cut end in vermiculite moistened with nutrient solution ( ± B, 0.1 mM), and photographed one week later (A). Using the same nutrient solution ( ± B, 0.1 mM), sterile pieces of the thallus of the liverwort Marchantia polymorpha were incubated on agar-plates. Five weeks later, in both treatments numerous rhizoids had developed on the lower side of the bryophyte (original experiments).
Since the earliest vascular plants, such as Z. shengfengense (), may have developed roots that we today would call “adventitious," it is possible that the micronutrient B played a major role during the evolutionary development of root systems, as suggested by Lewis.Citation1 As noted, the molecular mode of B-action is largely unknown.Citation21 However, additional functionalities for B that can affect root development have been discovered. For example, B deficiency is known to repress cytokinin receptor genes resulting in defective xylem differentiation,Citation22 results in leaf growth defects,Citation23 and may cause an induction of aquaporin-genes.Citation5 It also prevents rhamnogalacturon-II cross-linking by means of borate diesters, which form normally either intra-protoplasmically or during secretion.Citation24 Thus, B is critical to proper cell-to-cell signaling and adhesion, thereby influencing the mechanical properties of cells walls via the modulation of the metabolism of pectins.Citation25,26
Conclusions and outlook
Based on numerous experimental studies, we now know that B is involved in phytohormone signaling and influences the mechanical properties of intercellular pectins. From these data, we conclude that the role of Boron during the evolutionary development of lignified plant organs, i.e., roots and shoots,Citation1 should be explored in more detail to resolve root form vs. function relationships that are currently poorly understood.Citation27 Finally, we want to stress that root development is, under natural conditions, also regulated by epiphytic microbes that inhabit the rhizosphere.Citation4,28,29 The interaction between the micronutrient B and bacteria that live in (or are attached to) the root system is unknown. Hence, more integrative “plant-nutrients-microbe”-studies are required to resolve the puzzle of root development during the evolution of land plants.Citation2,3,7
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The cooperation of the authors is supported by the Alexander von Humboldt-Foundation, Bonn, Germany (AvH-Fellowship Stanford 2014 to U. K.).
References
- Lewis DH. Boron, lignification and the origin of vascular plants – a unified hypothesis. New Phytol 1980; 84:209-29; https://doi.org/10.1111/j.1469-8137.1980.tb04423.x
- Kenrick P, Strullu-Derrien C. The origin and early evolution of roots. Plant Physiol 2014; 166:570-80; PMID: 25187527; https://doi.org/10.1104/pp.114.244517
- Gerrienne P, Servais T, Vecoli M. Plant evolution and terrestrialization during Palaeozoic times. The phylogenetic context. Rev Paleobot Palynol 2016; 227:4-18; https://doi.org/10.1016/j.revpalbo.2016.01.004
- Klikno J, Kutschera U. Regulation of root development in Arabidopsis thaliana by phytohormone-secreting epiphytic methylobacteria. Protoplasma 2017; 1–11; https:doi.org/10.1007/s00709-016-1067-7
- Maurel C, Boursiac Y, Lun D-J, Santoni V, Shahzad Z, Verdoucq L. Aquaporins in plants. Physiol Rev 2015; 95:1321-58; PMID: 26336033; https://doi.org/10.1152/physrev.00008.2015
- Jones VAS, Dolan L. The evolution of root hairs and rhizoids. Ann Bot 2012; 110:205-12; PMID: 22730024; https://doi.org/10.1093/aob/mcs136
- Niklas KJ. Plant Evolution. An Introduction of the History of Life. University of Chicago Press 2016. Chicago, IL, USA.
- Kutschera U, Niklas KJ. Darwin-Wallace Demons: Survival of the fastest in populations of duckweeds and the evolutionary history of an enigmatic group of angiosperms. Plant Biol 2015; 17(Suppl. 1):24-32; PMID: 24674028; https://doi.org/10.1111/plb.12171
- Beck CB. An Introduction to Plant Structure and Development. Cambridge University Press, Cambridge. 2nd edition. 2005.
- Heimsch C, Seago Jr. JL. Organization of the root apical meristem in angiosperms. Am J Bot 2008; 95:1-21; PMID: 21632311; https://doi.org/10.3732/ajb.95.1.1
- Niklas KJ, Kutschera U. The evolutionary development of plant body plans. Funct Plant Biol 2009; 36:682-95; https://doi.org/10.1071/FP09107
- Niklas KJ, Kutschera U. The evolution of the land plant life cycle. New Phytol 2010; 185:27-41; PMID: 19863728; https://doi.org/10.1111/j.1469-8137.2009.03054.x
- Hao S, Xue J, Guo D, Wang D. Earliest rooting system and root: shoot ratio from a new Zosterophyllum plant. New Phytol 2010; 185:217-25; PMID: 19825018; https://doi.org/10.1111/j.1469-8137.2009.03056.x
- Schmucker T. Zur Blütenbiologie tropischer Nymphaea-Arten. II. (Bor als entscheidender Faktor.). Planta 1933; 18:641-50; https://doi.org/10.1007/BF01912645
- Schelp BJ. Physiology and biochemistry of boron in plants. In Boron and its role in crop production, pp. 53-85. Gupta UC, ed. Boca Raton, FL: CRC Press. 1993.
- Matoh T, Kobayashi MJ. Boron and calcium, essential inorganic constitutents of pectic polysaccharides in higher plant cell walls. Plant Res 1998; 111:179-90; https://doi.org/10.1007/BF02507164
- Middleton W, Jarvis BC, Booth A. The boron requirement for root development in stem cuttings of Phaseolus aureus Roxto. New Phytol 1978; 81:287-95; https://doi.org/10.1111/j.1469-8137.1978.tb02634.x
- Josten P, Kutschera U. The micronutrient boron causes the development of adventitious roots in sunflower cuttings. Ann Bot 1999; 84:337-42; https://doi.org/10.1006/anbo.1999.0922
- Kutschera U, Koopmann V. Growth in liverworts of the Marchantiales is promoted by epiphytic methylobacteria. Naturwissenschaften 2005; 92:347-49; PMID: 15965759; https://doi.org/10.1007/s00114-005-0640-2
- Matsunaga T, Ishii T, Matsumoto S, Higuchi M, Darill A, Albersheim P, O'Neill MA. Occurrence of the primary cell wall polysccharide Rhamnogalacturonan II in Pteridophytes, Lycophytes, and Bryophytes. Implications for the evolution of vascular plants. Plant Physiol 2004; 134:339-51; PMID: 14671014; https://doi.org/10.1104/pp.103.030072
- Gupta U, Solanki H. Impact of Boron deficiency on plant growth. Int J Bioassays 2013; 2:1048-50.
- Abreu I, Poza L, Bonilla I, Bolaños L. Boron deficiency results in early repression of a cytokinin receptor gene and abnormal cell differentiation in the apical root meristem of Arabidopsis thaliana. Plant Physiol Biochem 2014; 77:117-21; PMID: 24589475; https://doi.org/10.1016/j.plaphy.2014.02.008
- Liu G, Dong X, Liu L, et al. Boron deficiency is correlated with changes in cell wall structure that lead to growth defects in the leaves of navel orange plants. Scientia Horticulturae 2014; 176:54-62; https://doi.org/10.1016/j.scienta.2014.06.036
- Chormova D, Messenger DJ, Fry SC. Rhamnogalacturon-II cross-linking of plant pectins via boron bridges occus during polysaccharide synthesis and/or secretion. Plant Signal Behav 2014; 9:e28169; PMID: 24603547; https://doi.org/10.4161/psb.28169
- Iversen CM. Using root form to improve our understanding of root function. New Phytol 2014; 203:707-9; PMID: 25040729; https://doi.org/10.1111/nph.12902
- Edelmann HG, Wimmer MA, Goldbach HE. Immunogold-labelling of boric acid-binding sites by immunoreaction to FITC-moiety of FITC-boronic acid: First results. J. Trace and Microprobe Techniques 2000; 18:451-59.
- Kutschera U, Niklas KJ. Cell division and turgor-driven stem elongation in juvenile plants: A synthesis. Plant Sci 2013; 207:45-56; PMID: 23602098; https://doi.org/10.1016/j.plantsci.2013.02.004
- Kutschera U. Plant-associated methylobacteria as co-evolved phytosymbionts: A hypothesis. Plant Signal Behav 2007; 2:74-78; PMID: 19516971; https://doi.org/10.4161/psb.2.2.4073
- Kutschera U, Khanna R. Plant gnotobiology: Epiphytic microbes and sustainable agriculture. Plant Signal. Behav 2016; 11/12:e1256529, 1–4; https://doi.org/10.1080/15592324.2016.1256529