ABSTRACT
In plant cells, the vacuolar-type H+-ATPase (V-ATPase), a large multis`ubunit endomembrane proton pump, plays an important role in acidification of subcellular organelles, pH and ion homeostasis, and endocytic and secretory trafficking. V-ATPase subunit c (VHA-c) is essential for V-ATPase assembly, and is directly responsible for binding and transmembrane transport of protons. In previous studies, we identified a PutVHA-c gene from Puccinellia tenuiflora, and investigated its function in plant growth. Subcellular localization revealed that PutVHA-c is mainly localized in endosomal compartments. Overexpression of PutVHA-c enhanced V-ATPase activity and promoted plant growth in transgenic Arabidopsis. Furthermore, the activity of V-ATPase affected intracellular transport of the Golgi-derived endosomes. Our results showed that endomembrane localized-VHA-c contributes to plant growth by influencing V-ATPase-dependent endosomal trafficking. Here, we discuss these recent findings and speculate on the VHA-c mediated molecular mechanisms involved in plant growth, providing a better understanding of the functions of VHA-c and V-ATPase.
V-ATPase is a conserved proton pump found in eukaryotes, including yeasts, plants, and animals. V-ATPase consists of the cytosolic ATP-hydrolyzing V1 domain (subunits A to H) and the membrane-bound proton-translocating V0 domain (subunits a, c, c', c', d, and e).Citation1 The VHA-a containing three different isoforms is responsible for targeting V-ATPase to different subcellular sites in Arabidopsis.Citation2 V-ATPase complexes containing VHA-a1 are localized to the trans-Golgi network/early endosome (TGN/EE), whereas holoenzymes containing VHA-a2 or VHA-a3 are targeted to the tonoplast.Citation3 V-ATPase activity in the TGN/EE is required for endocytic and secretory trafficking, whereas V-ATPase activity at the tonoplast is required for vacuolar ion homeostasis and efficient nutrient storage.Citation4,5
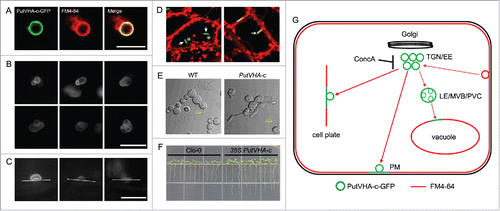
The V-ATPase c subunit (VHA-c), which includes four transmembrane helices, is a highly hydrophobic protein in the V0 domain.Citation1 In a previous study, we identified a highly conserved VHA-c gene (PutVHA-c) from Puccinellia tenuiflora. Compared to the wild-type, transgenic Arabidopsis plants overexpressing PutVHA-c exhibit better growth phenotypes in root length, fresh weight, plant height, and silique number under normal conditions due to noticeably higher V-ATPase activity. Confocal and immunogold electron microscopy assays demonstrate that PutVHA-c is mainly localized to the endosomal compartments. Concanamycin A (ConcA) is a membrane-permeable macrolide antibiotic that specifically binds to VHA-c and inhibits proton transport. ConcA treatment resulted in accumulation of endosomes containing PutVHA-c.Citation6 Previous studies showed that ConcA induces the aggregation of large Golgi-derived endosomes.Citation3 These findings suggest that PutVHA-c-containing endosomes may be derived from the Golgi apparatus, and that endosomal trafficking was affected by V-ATPase activity. In addition, we found that endosomes containing PutVHA-c can fuse with each other (, ), and can simultaneously transport and fuse with the plasma membrane, tonoplast, and cell plate (, ), suggesting that PutVHA-c is distributed throughout the secretory pathway. Phenotype experiments showed that overexpression of PutVHA-c can promote cell expansion in yeast and Arabidopsis seedling roots (, ). Membranes for cell growth, membrane proteins, extracellular proteins, and components of the cell wall, which are necessary for plant cell growth, are transported to target sites via the endosome-mediated trafficking.Citation5,7,Citation8 Direct evidence shows that the reduced V-ATPase activity in the TGN/EE causes hypocotyl cell expansion inhibition by affecting the synthesis and trafficking of cell wall components.Citation9 Therefore, the endosome-mediated transport of the components necessary for cell growth is likely related to plant cell expansion. As such, we hypothesized that overexpression of PutVHA-c may accelerate endosomal trafficking by enhancing V-ATPase activity, thereby promoting plant cell expansion (). In the future, isolation of PutVHA-c localized-endosomes, and further identification of the components in endosomes will be essential.
Figure 1. Expression and localization of PutVHA-c in Arabidopsis and yeast cells. (A) PutVHA-c colocalizes with FM4-64 in the endosomal compartment. (B and C) Endosomes containing PutVHA-c fuse with each other (B) and with the plasma membrane (C). (D) PutVHA-c partially localized to the tonoplast (indicated with an arrow, in the left plate) and to the stump of the cell plate (indicated with an arrow, in the right plate) labeled by FM4-64. The asterisk indicates the vacuole. (E and F) Expression of PutVHA-c promotes cell expansion in yeast (E) and Arabidopsis root (F). (G) A summary of the localization of PutVHA-c in plant cells. TGN/EE, trans-Golgi network/early endosome; LE/MVB/PVC, late endosomes/multivesicular body/prevacuolar compartment. PM, plasma membrane. ConcA, Concanamycin A.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Forgac M. Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat rev Mol Cell Biol. 2007;8:917–929. doi:10.1038/nrm2272.
- Schumacher K, Krebs M. The V-ATPase: small cargo, large effects. Curr Opin Plant Biol. 2010;13:724–730. doi:10.1016/j.pbi.2010.07.003.
- Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumacher K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell. 2006;18:715–730. doi:10.1105/tpc.105.037978.
- Krebs M, Beyhl D, Gorlich E, Al-Rasheid KA, Marten I, Stierhof YD, et al. Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proc Natl Acad Sci USA. 2010;107:3251–3256. doi:10.1073/pnas.0913035107.
- Luo Yu, Schumacher K. V-ATPase activity in the TGN/EE is required for exocytosis and recycling in Arabidopsis. Nat plants. 2015;1:15094. doi:10.1038/nplants.2015.94.
- Zhou A, Bu Y, Takano T, Zhang X, Liu S. Conserved V-ATPase c subunit plays a role in plant growth by influencing V-ATPase-dependent endosomal trafficking. Plant Biotech J. 2016;14:271–283. doi:10.1111/pbi.12381.
- Battey NH, Blackbourn HD. The control of exocytosis in plant cells. New Phytol. 1993;125:307-338. doi:10.1111/j.1469-8137.1993.tb03883.x.
- Robinson DG, Pimpl P. Clathrin and post-Golgi trafficking: a very complicated issue. Trends Plant Sci. 2014;19:134–139. doi:10.1016/j.tplants.2013.10.008.
- Brüx A, Liu TY, Krebs M, Stierhof YD, Lohmann JU, Miersch O, Wasternack C, Schumacher K. Reduced V-ATPase activity in the trans-Golgi network causes oxylipin-dependent hypocotyl growth Inhibition in Arabidopsis. Plant Cell. 2008;20:1088–1100 doi:10.1105/tpc.108.058362.
