ABSTRACT
Perception of the plant hormone jasmonoyl-isoleucine (JA-Ile) involves the formation of a co-receptor complex between COI1, the F-box subunit of a SCF-type E3 ubiquitin ligase, and its substrates, a group of jasmonate ZIM-domain (JAZ) transcriptional repressors. In recent studies, we show that MED25, a subunit of the Arabidopsis Mediator, physically and functionally interacts with COI1 and the master transcription factor MYC2 on MYC2 target promoters. Here we provide evidence that MED25 also physically interacts with a subset of JAZ proteins. Therefore, in term of their interaction with Mediator, the JA-Ile co-receptor complex SCFCOI1-JAZs together with the master transcription factor MYC2 resembles the nuclear hormone receptor system of metazoans. In addition, we show that the plant MED25 and its animal counterpart also cooperates with similar epigenetic regulators in distinct signaling pathways. Our study reveals a scenario that plants and animals have evolved distinct, yet largely similar, mechanism for nuclear hormone receptor activation at the level of transcriptional regulation.
The striking capacity for plants to adapt their growth and development to the ever-changing environment is mediated by diverse plant hormones that regulate virtually every aspect of plant life.Citation1 In the past 10–15 years, enormous progress has been made in elucidating the nature of plant hormone receptors. An important conceptual advance in hormone biology is the finding that, in addition to the conventional membrane-bound hormone receptors, the receptors of several major plant hormones including auxin,Citation2 jasmonate,Citation3,4 gibberellins,Citation5 abscisic acid,Citation6 and salicylic acid,Citation7 are localized in the nucleus and directly linked to hormone-regulated gene transcription. Despite these progresses, the physiological significance and action mechanism of nuclear hormone receptors (NRs) in the plant kingdom remain enigmatic.
Jasmonate (JA) is a lipid-derived plant hormone that regulates a wide range of plant immunity and adaptive growth.Citation8–10 Sensing of jasmonoyl-isoleucine (JA-Ile), the active form of JA, by the nuclear-localized F-box protein CORONATINE INSENSITIVE 1 (COI1) triggers genome-wide transcriptional changes that are largely regulated by the master transcription factor MYC2.Citation11–13 In a recent study, we showed that Mediator, an evolutionarily conserved multi-subunit coactivator complex whose activity is essential for RNA polymerase II (Pol II)-dependent gene transcription, directly links COI1 to Pol II and chromatin during JA signaling.Citation14 We found that, in the resting stage, the Mediator subunit MED25 brings COI1 to MYC2 target promoters through physical interaction. Upon hormone elicitation, MED25 facilitates COI1-dependent degradation of jasmonate ZIM-domain (JAZ) transcriptional repressors, which favors MYC2-directed transcription of JA-responsive genes.
These new observations, together with our previous finding that MED25 bridges the master transcription factor MYC2 and Pol II for pre-initiation complex (PIC) assembly during JA-regulated gene transcription,Citation15 reveal that MED25 links the receptor and the master transcription factor of JA signaling through direct interaction. We also showed that MED25 could be coimmunoprecipitated by JAZ1,Citation14 which is another major component of the SCFCOI1-JAZ co-receptor complex.Citation4,16,17 To further test whether MED25 interacts with the JAZ family proteins directly, we performed yeast two-hybrid (Y2H) assays and found that MED25 showed relatively strong interaction with JAZ1 and JAZ3 while showed weak interaction with JAZ2 (). These results suggested that MED25 interacts with subsets of JAZ proteins. In firefly luciferase (LUC) complementation imaging (LCI) assays, Nicotiana benthamiana cells co-transformed with MED25-nLUC and cLUC-JAZ1 showed strong fluorescence signals, whereas those co-transformed with MED25-nLUC and cLUC or cLUC-JAZ1 and nLUC showed no fluorescence signal (). These results confirmed that MED25 interacts with JAZ1 in plant cells. Domain mapping with LCI assays showed that the glutamine-rich domain (GD) of MED25 and the ZIM domain of JAZ1 are involved in MED25-JAZ1 interaction ( and ). Taken together, our results indicate that MED25 directly interacts with the receptor COI1, the co-receptor JAZ proteins and the master transcription factor MYC2 in JA signaling.
Figure 1. MED25 interacts with a subset of JAZ proteins. (A) Y2H assays show the interaction of MED25 with JAZ proteins. Transformed yeast strains were plated on SD medium lacking His, Ade, Leu, and Trp (SD/−4). (B) MED25 interacts with JAZ1 in LCI assays. (Top) LUC images of N. benthamiana leaves coinfiltrated with the different construct combinations are shown in the lower quadrant of the circle. (C) and (D) Mapping of the protein domains involved in MED25-JAZ1 interaction using LCI assays. (C) Based on the schematic protein structure of MED25, full-length MED25 or its derivatives (MED25-nLUC or MED25-nLUC derivatives) were tested for interactions with JAZ1 (cLUC-JAZ1). N. benthamiana leaves co-transformed with MED25-nLUC or MED25-nLUC derivatives and cLUC-JAZ1 were imaged 72 h after Agrobacterium infiltration. (D) Based on the schematic protein structure of JAZ1, full-length JAZ1 or its derivatives (cLUC-JAZ1 or cLUC-JAZ1 derivatives) were tested for interaction with MED25 (MED25-nLUC). N. benthamiana leaves co-transformed with cLUC-JAZ1 or cLUC-JAZ1 derivatives and MED25-nLUC were imaged 72 h after Agrobacterium infiltration.
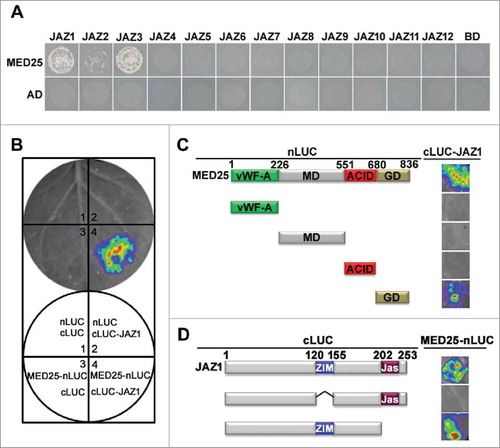
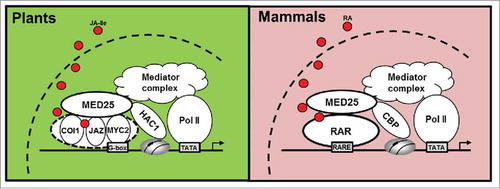
Figure 2. Analogy of nuclear hormone receptor activation system between plants and mammals. The “COI1-JAZ-MYC2” module of JA signaling in plants resembles the nuclear hormone receptor RAR in mammals. In plants, MED25 interacts with COI1 and JAZ proteins and facilitates JA-Ile-triggered degradation of JAZ proteins, which in turn enhances the MED25-MYC2 interaction. MED25 functions in chromatin modification and preinitiation complex assembly by recruiting HAC1 and the Mediator complex, respectively, to MYC2-targeted promoters. In mammals, MED25 interacts with RAR in the presence of RA and functions in chromatin modification and preinitiation complex by recruiting CBP1 and the Mediator complex, respectively, to RAR-responsive promoters.
In addition, we found that MED25 also physically and functionally interacts with HISTONE ACETYLTRANSFERASE1 (HAC1), an evolutionarily conserved histone modification enzyme that selectively regulates histone (H) 3 lysine (K) 9 acetylation (H3K9ac) of MYC2 target promoters, during JA signaling. Moreover, MED25 cooperates with both COI1 and HAC1 on MYC2 target promoters.Citation14 Our results reveal that MED25 acts as a master coordinator to integrate the actions of both genetic and epigenetic regulators into a concerted transcriptional program.
Significantly, the above-described action mode of MED25 in regulating JA signaling shows striking analogy to that of its mammalian counterpart, which engages in a ligand-dependent interaction with the retinoic acid receptor (RAR) and several other NRs.Citation18 Animal NRs are themselves ligand-activated transcription factors which comprise a conserved DNA binding domain (DBD) that mediates specific hormone response element (HRE) recognition and a ligand-binding domain (LBD) that is essential for ligand-dependent transcriptional activation.Citation19 Upon binding to retinoic acid (RA), RAR undergoes a significant conformational change to create a hydrophobic binding surface suitable for the interaction with human MED25. In addition, during RAR activation the human MED25 also interacts and cooperates with CREB bind protein (CBP), a human ortholog of the Arabidopsis HAC1.Citation18 Therefore, in term of their interaction with MED25, the SCFCOI1-JAZ co-receptor complex together with the master transcription factor MYC2 resembles the NR system of metazoans. In both systems, hormone-dependent protein interactions is a conserved strategy governing NR activation (). Thus, our study reveals a scenario in which plants and animals have evolved distinct, but nonetheless largely similar, mechanisms for NR activation at the level of transcriptional regulation.
Moreover, the signaling paradigm that Mediator links hormone receptor to transcriptionally active chromatin is likely operates in other plant hormones whose receptors are localized in the nucleus. Our findings lay important foundation not only to investigate the action of Mediator in other plant hormones and but also to examine the similarity and difference between plant and animal NR systems.
Funding
This work was supported by the National Natural Science Foundation of China [Grant No. 31730010]; the Youth Innovation Promotion Association CAS; and the Tai-Shan Scholar Program from the Shandong Provincial Government.
References
- Smith MS, Li C, Li J. Hormone function in plants. In: Li J, Li C, Smith MS, editors. Hormone metabolism and signaling in plants. London (United Kingdom): ELSEVIER Academic Press; 2017. p. 1–38.
- Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–5. doi:10.1038/nature03543.
- Yan J, Zhang C, Gu M, Bai Z, Zhang W, Qi T, Cheng Z, Peng W, Luo H, Nan F, et al. The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell. 2009;21:2220–36. doi:10.1105/tpc.109.065730.
- Sheard LB, Tan X, Mao H, Withers J, Ben-Nissan G, Hinds TR, Kobayashi Y, Hsu FF, Sharon M, Browse J, et al. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature. 2010;468:400–5. doi:10.1038/nature09430.
- Shimada A, Ueguchi-Tanaka M, Nakatsu T, Nakajima M, Naoe Y, Ohmiya H, Kato H, Matsuoka M. Structural basis for gibberellin recognition by its receptor GID1. Nature. 2008;456:520–3. doi:10.1038/nature07546.
- Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–71.
- Fu ZQ, Yan S, Saleh A, Wang W, Ruble J, Oka N, Mohan R, Spoel SH, Tada Y, Zheng N, et al. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature. 2012;486:228–32.
- Browse J. Jasmonate passes muster: a receptor and targets for the defense hormone. Annu Rev Plant Biol. 2009;60:183–205. doi:10.1146/annurev.arplant.043008.092007.
- Wasternack C, Hause B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot. 2013;111:1021–58. doi:10.1093/aob/mct067.
- Zhai Q, Yan C, Li L, Xie D, Li C. Jasmonates. In: Smith MS, Li C, Li J, editors. Hormone metabolism and signlaing in plants. London (United Kingdom): ELSEVIER Academic Press; 2017. p. 243–72.
- Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, Miersch O, Wasternack C, Solano R. (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nature Chem Biol. 2009;5:344–50. doi:10.1038/nchembio.161.
- Devoto A, Nieto-Rostro M, Xie D, Ellis C, Harmston R, Patrick E, Davis J, Sherratt L, Coleman M, Turner JG. COI1 links jasmonate signalling and fertility to the SCF ubiquitin-ligase complex in Arabidopsis. Plant J. 2002;32:457–66. doi:10.1046/j.1365-313X.2002.01432.x.
- Kazan K, Manners JM. MYC2: the master in action. Mol Plant. 2013;6:686–703. doi:10.1093/mp/sss128.
- An C, Li L, Zhai Q, You Y, Deng L, Wu F, Chen R, Jiang H, Wang H, Chen Q, et al. Mediator subunit MED25 links the jasmonate receptor to transcriptionally active chromatin. Proc Natl Acad Sci USA. 2017;114(42):E8930–39. doi:10.1073/pnas.1710885114.
- Chen R, Jiang H, Li L, Zhai Q, Qi L, Zhou W, Liu X, Li H, Zheng W, Sun J, et al. The Arabidopsis mediator subunit MED25 differentially regulates jasmonate and abscisic acid signaling through interacting with the MYC2 and ABI5 transcription factors. Plant Cell. 2012;24:2898–916. doi:10.1105/tpc.112.098277.
- Chini A, Fonseca S, Fernandez G, Adie B, Chico JM, Lorenzo O, García-Casado G, López-Vidriero I, Lozano FM, Ponce MR, et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007;448:666–71. doi:10.1038/nature06006.
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature. 2007;448:661–5. doi:10.1038/nature05960.
- Lee HK, Park UH, Kim EJ, Um SJ. MED25 is distinct from TRAP220/MED1 in cooperating with CBP for retinoid receptor activation. EMBO J. 2007;26:3545–57. doi:10.1038/sj.emboj.7601797.
- Fondell JD. The Mediator complex in thyroid hormone receptor action. Biochim Biophys Acta. 2013;1830:3867–75. doi:10.1016/j.bbagen.2012.02.012.
