ABSTRACT
Endomembrane protein trafficking has emerged as important means of regulating stress responses in plants. The Arabidopsis SNX1 protein is involved in recycling the iron transporter IRT1, thus promoting its presence at the plasma membrane. SNX1 and its interacting partners undergo stress-related regulation at both transcriptional and posttranslational level, which may include differential regulation at tissue level. Based on this, we explore the tissue-specific regulation of iron import, specifically concentrating on the factors involved in the expression and recycling of IRT1 in root tissues. We propose that different processes affecting IRT1 regulation may lead to similar outcomes, allowing for fine-tuning iron acquisition and distribution.
SORTING NEXINs (SNX) are proteins present in all eukaryotic organisms and implicated in the regulation of endomembrane protein trafficking.Citation1 In Arabidopsis, the SNX1 protein belongs to a six-member protein family and is involved, among others, in the recycling of plasma membrane transporters that results in their retargeting to the plasma membrane.Citation2 One of the SNX1 targets is the IRON-REGULATED TRANSPORTER1 (IRT1), responsible for the acquisition of iron from the rhizosphere. In the absence of SNX1, IRT1 is preferentially targeted for degradation, leading to a reduced capacity of Arabidopsis to acquire iron under iron limited conditions.Citation3
At present, it is unclear how the composition, and therefore the activity, of SNX1-containing protein complexes is regulated on cellular level and in response to stress. In this context, we used stress-related gene expression and phosphoproteomic data to analyze the transcriptional, and post-translational regulation of SNX1, together with all SNX1-interacting Arabidopsis proteins.Citation4 Our analysis revealed that the genes encoding SNX1 interactors respond to different stresses through specific spaciotemporal expression patterns, suggesting that the composition of SNX1 complexes is cell-specific. Upon perception of external stimulus, SNX1 may change its protein environment, potentially affecting SNX1-dependent endomembrane protein recycling. We could conclude that stress-related phosphorylation of SNX1-interacting proteins occurs complementary to the transcriptional regulation of the genes encoding these proteins.Citation4 Thus, the stress responsiveness of the SNX1 interactors is subject to two layers of regulation complexity – expression control in space and time, and activity modulation though transcriptional or post-translational mechanisms. Therefore, through engaging with SNX1 in a protein complex, the stress responsiveness of these interactors translates to a stress-responsive SNX1 protein sorting activity.
The dataset on SNX1 interactors’ regulation provides three important pieces of information. First, a full list of manually-curated currently-known interactions of the Arabidopsis SNX1, together with a model placing these interactions in a cell-biological context. Second, it sheds light on the transcriptional regulation together with phosphorylation events affecting protein trafficking, two emerging topics of great significance for future systems-level analyses of endomembrane trafficking processes. Third, it offers an additional perspective on the events underlying the tissue-level regulation of iron acquisition.
Concerning the third point, recent studies have shown that the expression of the IRT1 gene falls under tight positional regulation in the root in both longitudinal and lateral directions. It was shown that IRT1 expression is highest in the early root differentiation zone.Citation5,Citation6 Histochemical studies visualizing IRT1 promoter activity showed that this is a result of the dynamic interaction between ethylene- and auxin-mediated events, likely in response to iron availability. Ethylene was found to promote the expression of IRT1 in the early root differentiation zone,Citation7 while auxin prompted its exclusion from there ().Citation7,Citation8
Figure 1. A hypothetical model for the cell-specific regulation of IRT1 expression and protein recycling under iron deficiency. (A) IRT1 is expressed in the early differentiation zone of the root. Its expression domain is defined by the opposing effects of the phytohormones ethylene and auxin. (B) In the central cylinder, FIT activity is inhibited through its interaction with ZAT12. In the absence of reactive oxygen species (ROS), ZAT12 is unstable and is degraded through a non-proteasomal pathway. This releases potentially active FIT. SNX1 expression is upregulated in this zone (green arrow), enhancing the cellular capacity to recycle SNX1 target proteins. In the epidermis, FIT can engage in protein complexes with ZAT12 but also with DELLA proteins, such as RGA. In these cells, ZAT12 undergoes proteasome-mediated degradation. Under iron deficiency, the phytohormone gibberellin (GA) promotes the proteasomal degradation of DELLA. Both events may be mediated through the COP9 signalosome (not depicted) and result in the release of potentially active FIT. The gene encoding the SNX1 interactor BLOS1, promoting the vacuolar degradation of membrane proteins, is downregulated in the epidermis (red blunt line), thereby increasing the SNX1 potential for protein recycling. (C) Under iron deficiency, active FIT in both central cylinder and the epidermis promotes the expression of IRT1. The IRT1 protein is targeted to the plasma membrane for iron uptake (black punctate arrows). Endocytosis (blue punctate arrows) may lead to IRT1 degradation in the vacuole. Alternatively, endosomal IRT1 may be recycled and sent back to the plasma membrane (red punctate arrows). Accumulation of active SNX1 promotes IRT1 stability and increased plasma membrane abundance.
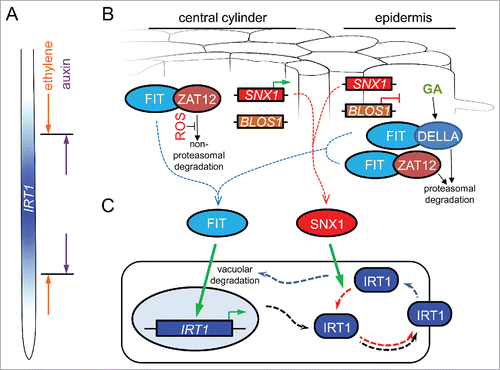
Regulated expression of IRT1 in radial root zones is crucial for iron homeostasis. Absence of IRT1 from either the central cylinder or the epidermis disrupts the capacity of plants to utilize iron.Citation9 IRT1 expression changes in response to a variety of environmental stimuli.Citation10 The main regulator of IRT1 expression under iron starvation is the transcription factor FIT,Citation11,Citation12 which interact with, among others, ZAT12 and the DELLA-family transcription factors. Both these interactions were suggested to negatively impact FIT function by depleting it from active complexes.Citation13,Citation14 In the central cylinder, ZAT12 stability is reactive oxygen species (ROS)-dependent, while its availability in the epidermis is proteasome-dependent ().Citation13,Citation15 The DELLA protein RGA was shown to be degraded in the epidermis of the root differentiation zone under iron deficiency, releasing its inhibition of FIT ().Citation14 The selection mechanism triggering this proteasome-dependent degradation of ZAT12 and RGA is at present unclear, however a recent study revealed that a large subset of the iron-responsive genes are deregulated in mutants of the COP9 signalosome.Citation16 The COP9 complex functions as an inhibitor of Cullin-RING E3 Ubiquitin ligases, thus influencing proteasome-mediated degradation of proteinsCitation17 and its role has already been demonstrated for the stability of the rice (Oryza sativa) iron acquisition transcription factor IDEF1.Citation18
Thus, iron deficiency causes the activation of FIT throughout the root, however the effects are based on different, radial zone-specific underlying mechanisms (, ).
The data on the expression of SNX1 and the genes encoding its known interaction partners suggests that the post-translational regulation of IRT1 may also differ between the central cylinder and the epidermis. In the central cylinder, iron deficiency causes the upregulation of SNX1 expression,Citation6 consistent with the increased abundance of SNX1-GFP protein in SNX1pro:SNX1-GFP lines.Citation3 This suggests an enhanced cellular potential for SNX1-dependent endosomal protein recycling in the central cylinder. In the epidermis, the expression of SNX1 is not upregulated by iron starvation. However, the gene encoding BLOS1, a SNX1 interactor promoting vacuolar degradation of plasma membrane transporters,Citation19 is downregulated,Citation6 potentially leading to an increase in SNX1 recycling capacity ().
Under iron deficiency, independent root zone-specific strategies, one in the central cylinder and one in the epidermis, may act to regulate the amount of IRT1 and maintain the cellular potential to support an increased pool of IRT1 at the plasma membrane. Such a scenario also suggests the possibility for fine-tuning iron acquisition and homeostasis by independently adjusting the IRT1 expression and protein stability in different cell types. This model illustrates an example of a coordinated action at different regulatory levels, a strategy which is commonly employed for achieving concerted stress responses in plants.Citation20 Investigating tissue-specific regulation of stress response will be crucial for understanding how this fine-tuning is achieved and how it could potentially be exploited in biotechnology and agriculture for the generation of stress-resistant crops.
Disclosure of potential conflicts of interest
Authors declare no conflicts of interest.
Acknowledgements
The research on endomembrane trafficking and plant stress is supported by the Heinrich Heine University.
Additional information
Funding
References
- Cullen PJ. Endosomal Sorting and Signalling: An Emerging Role for Sorting Nexins. Nat Rev Mol Cell Biol. 2008;9:574–82. doi:10.1038/nrm2427.
- Heucken N, Ivanov R. The Retromer, Sorting Nexins and the Plant Endomembrane Protein Trafficking. J Cell Sci. 2017. doi:10.1242/jcs.203695.
- Ivanov R, Brumbarova T, Blum A, Jantke AM, Fink-Straube C, Bauer P. SORTING NEXIN1 Is Required for Modulating the Trafficking and Stability of the Arabidopsis IRON-REGULATED TRANSPORTER1. Plant Cell. 2014;26:1294–307. doi:10.1105/tpc.113.116244.
- Brumbarova T, Ivanov R. Differential Gene Expression and Protein Phosphorylation as Factors Regulating the State of the Arabidopsis SNX1 Protein Complexes in Response to Environmental Stimuli. Front Plant Sci. 2016;7:1456. doi:10.3389/fpls.2016.01456.
- Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat JF, Curie C. IRT1, an Arabidopsis Transporter Essential for Iron Uptake from the Soil and for Plant Growth. Plant Cell. 2002; 14:1223–33. doi:10.1105/tpc.001388.
- Dinneny JR, Long TA, Wang JY, Jung JW, Mace D, Pointer S, Barron C, Brady SM, Schiefelbein J, Benfey PN. Cell Identity Mediates the Response of Arabidopsis Roots to Abiotic Stress. Science. 2008;320:942–5. doi:10.1126/science.1153795.
- Blum A, Brumbarova T, Bauer P, Ivanov R. Hormone Influence on the Spatial Regulation of IRT1 Expression in Iron-Deficient Arabidopsis thaliana Roots. Plant Signal Behav. 2014;9:e28787. doi:10.4161/psb.28787.
- Seguela M, Briat JF, Vert G, Curie C. Cytokinins Negatively Regulate the Root Iron Uptake Machinery in Arabidopsis Through a Growth-Dependent Pathway. Plant J. 2008;55:289–300. doi:10.1111/j.1365-313X.2008.03502.x.
- Marques-Bueno MM, Morao AK, Cayrel A, Platre MP, Barberon M, Caillieux E, Colot V, Jaillais Y, Roudier F, Vert G, et al. A Versatile Multisite Gateway-Compatible Promoter and Transgenic Line Collection for Cell Type-Specific Ffunctional Genomics in Arabidopsis. Plant J. 2016;85:320–33. doi:10.1111/tpj.13099.
- Brumbarova T, Bauer P, Ivanov R. Molecular Mechanisms Governing Arabidopsis Iron Uptake. Trends Plant Sci. 2015;20:124–133. doi:10.1016/j.tplants.2014.11.004.
- Colangelo EP, Guerinot ML. The Essential Basic Helix-Loop-Helix Protein FIT1 is Required for the Iron Deficiency Response. Plant Cell. 2004;16:3400–12. doi:10.1105/tpc.104.024315.
- Jakoby M, Wang HY, Reidt W, Weisshaar B, Bauer P. FRU (BHLH029) is Required for Induction of Iron Mobilization Genes in Arabidopsis thaliana. FEBS Lett. 2004;577:528–34. doi:10.1016/j.febslet.2004.10.062.
- Le CT, Brumbarova T, Ivanov R, Stoof C, Weber E, Mohrbacher J, Fink-Straube C, Bauer P. ZINC FINGER OF ARABIDOPSIS THALIANA12 (ZAT12) Interacts with FER-LIKE IRON DEFICIENCY-INDUCED TRANSCRIPTION FACTOR (FIT) Linking Iron Deficiency and Oxidative Stress Responses. Plant Physiol. 2016;170:540–57. doi:10.1104/pp.15.01589.
- Wild M, Daviere JM, Regnault T, Sakvarelidze-Achard L, Carrera E, Lopez Diaz I, Cayrel A, Dubeaux G, Vert G, Achard P. Tissue-Specific Regulation of Gibberellin Signaling Fine-Tunes Arabidopsis Iron-Deficiency Responses. Dev Cell. 2016;37:190–200. doi:10.1016/j.devcel.2016.03.022.
- Brumbarova T, Le CT, Ivanov R, Bauer P. Regulation of ZAT12 Protein Stability: The Role of Hydrogen Peroxide. Plant Signal Behav. 2016;11:e1137408. doi:10.1080/15592324.2015.1137408.
- Eroglu S, Aksoy E. Genome-Wide analysis of Gene Expression Profiling Revealed that COP9 Signalosome is Essential for Correct Expression of Fe Homeostasis Genes in Arabidopsis. Biometals. 2017;30:685–98. doi:10.1007/s10534-017-0036-8.
- Nezames CD, Deng XW. The COP9 Signalosome: Its Regulation of Cullin-Based E3 Ubiquitin Ligases and Role in Photomorphogenesis. Plant Physiol. 2012;160:38–46. doi:10.1104/pp.112.198879.
- Tan S, Liu F, Pan XX, Zang YP, Jin F, Zu WX, Qi XT, Xiao W, Yin LP. CSN6, a Subunit of the COP9 Signalosome, is Involved in Early Response to Iron Deficiency in Oryza sativa. Sci Rep. 2016;6:25485. doi:10.1038/srep25485.
- Cui Y, Li X, Chen Q, He X, Yang Q, Zhang A, Yu X, Chen H, Liu N, Xie Q, et al. BLOS1, a Putative BLOC-1 Subunit, Interacts with SNX1 and Modulates Root Growth in Arabidopsis. J Cell Sci. 2010;123:3727–33. doi:10.1242/jcs.069732.
- Haak DC, Fukao T, Grene R, Hua Z, Ivanov R, Perrella G, Li S. Multilevel Regulation of Abiotic Stress Responses in Plants. Front Plant Sci. 2017;8:1564. doi:10.3389/fpls.2017.01564.
