ABSTRACT
In Arabidopsis, aluminum (Al) exclusion from the root is mainly facilitated by Al-activated root malate and citrate exudation through the ALMT1 malate transporter and the MATE citrate transporter, respectively. However, the nature of an internal Al tolerance mechanism remains largely unknown. In a recent study, we showed that NIP1;2 facilitates Al-malate transport from the root cell wall into the root symplasm and subsequent root-to-shoot translocation and thus NIP1;2 plays key roles in Al detoxification and internal tolerance in Arabidopsis. We discovered that the NIP1;2-mediated Al removal from the root cell wall requires a functional ALMT1-mediated malate exudation system, which allows the formation of an Al-malate complex in the root cell wall. Thus, a coordinated function between the exclusion and the internal resistance mechanisms, linked by the ALMT1-mediated root malate exudation and the NIP1;2-mediated Al uptake system, is critical for Al resistance in Arabidopsis.
In acid soils (pH < 5), toxic aluminum (Al) ions, Al3+, are released from aluminosilicate clays into the soil solution, causing root growth inhibition and limited nutrient and water uptake by plants.Citation1 As acid soils are widely distributed around the world, Al toxicity is a major constraint that limits crop yields on acid soils worldwide.Citation1-4
Plants have evolved several Al resistant mechanisms, including (1) a well characterized exclusion mechanism, through which plants release organic acids (OAs), e.g., malate and citrate, or other organic ligands from the root into the rhizosphere, which prevents toxic Al3+ ions from entering into the root cells; (2) a less well characterized internal tolerance mechanism through which the toxic cytosolic Al in the root is sequestered to the vacuole of the root cell and/or translocated to the shoot for further sequestration in the leaf vacuole.Citation2,Citation3,Citation5
Over the past decade, key components involved in the Al exclusion mechanism have been identified in different plant species.Citation6 A unique feature for the cloned Al resistance genes is that they commonly encode plasma-membrane (PM) localized malate or citrate transporters from the Al-activated malate transporter (ALMT) and the multidrug and toxic compound extrusion (MATE) families and facilitate Al-activated malate and citrate exudation, respectively, in wheat, sorghum, barley, maize and Arabidopsis.Citation7-13 In Arabidopsis thaliana, transcriptional expression of ALMT1 and MATE is controlled by a master zinc-finger transcription factor, STOP1.Citation11,14,15 Recently we have identified several stop1 suppressor mutants, which could help reveal the STOP1-mediated signaling pathways involved in low pH and Al resistance in Arabidopsis.Citation16
In contrast, the internal Al resistance mechanism is less well characterized in plants. Recent studies identified OsNrat1 (Nramp aluminum transporter 1) as a putative Al transporter involved in the internal resistance mechanism in rice that lowers Al concentrations in the root cell wall.Citation17,Citation18
Aluminum resistance in Arabidopsis
In Arabidopsis, the Al exclusion mechanism is mainly achieved by Al-activated malate and citrate exudation from the root.Citation8, Citation11 ALMT1 is expressed in the root tip region and is responsible for a large amount of Al-activated malate releases from the root tip upon Al stress, while MATE plays a smaller but significant role in Al resistance, responsible for releases of a smaller amount of citrate from the mature root region.Citation12
Aluminum retained in the root cell wall is toxic to plants
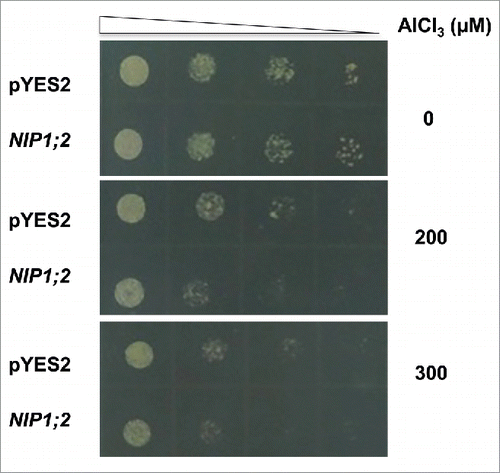
Increasing evidence has demonstrated that the root cell wall in the root tip region is a major target for Al toxicity in plants.Citation19,Citation20 Our recent studies indicated that even with a functional Al-activated OA exudation system that prevents Al from entering the root cell, the simple binding of Al and malate is not enough to provide full protection.Citation21 The Al-malate complexes in the cell wall need to be removed from the root tip region to reach a higher level of Al resistance.Citation21 We have demonstrated that NIP1;2, a PM-localized member of the nodolin 26-like intrinsic protein (NIP) subfamily of the aquaporin (AQP) superfamily, functions as an Al-malate (Al-Mal) transport that facilitates Al-Mal transport from the root cell wall into the root cytosol in the root tip region, and subsequent root-to-shoot Al translocation in Arabidopsis.Citation21 NIP1;2 is expressed in the root tip and this expression is enhanced by Al stress.Citation21 When heterologously expressed in Saccharomyces cerevisiae (BY4741 strain), NIP1;2 facilitated Al-Mal transport across the PM.Citation21 Under a control condition (-Al), no growth difference could be observed between a control yeast line transformed with an empty vector and an NIP1;2-expressing line (), suggesting that heterologously expressed NIP1;2 was not harmful for normal yeast growth. However, when supplied with 200 or 300 μM Al-malate, the NIP1;2-expressing yeast line displayed a hyper-sensitive phenotype to Al stress (). Such a growth inhibition can be explained by the NIP1;2-facilitated Al accumulation in the yeast cells.Citation21
Figure 1. NIP1;2-expressing yeast cells are hyper-sensitive to Al stress. Yeast cells (BY4741) carrying an empty vector (pYES2) or a pYES2-NIP1;2 (NIP1;2) construct were cultured in a liquid SD-Ura medium to a stationary phase before collected by centrifugation and then washed 3 times with deionized water. Yeast cells then underwent four 10-fold serial dilutions with a low pH, low magnesium (LPM) medium (pH 4.2). Then, 5 μL of each dilution sample was spotted onto LPM plates containing 0, 200 or 300 μM AlCl3 supplied with 2% galactose for induction of the GAL promoter, 300 mM malate and buffered with 5 mM succinic acid at pH 4.2, and. The LPM plates were placed in a 30oC incubator for 3 d.
Al-activated root organic acid exudation is independent of NIP1;2 function
In Arabidopsis, an almt1 knockout (KO) mutation completely abolishes the ALMT1-mediated root malate exudation upon Al stress and thus the corresponding almt1-KO mutant is extremely hypersensitive to Al toxicity.Citation8 In our recent study, we demonstrated that under Al stress, almt1-KO was also defective in removing Al from the root cell wall in spite of the fact that this mutant has a functional NIP1;2.21 Interestingly, when malate was externally supplied, the NIP1;2-mediated Al removal from the root cell wall was restored in almt1-KO, indicating that ALMT1-mediated root malate exudation is a prerequisite for the NIP1;2-mediated Al-Mal transport into the cytosol of the root cell in Arabidopsis.Citation21
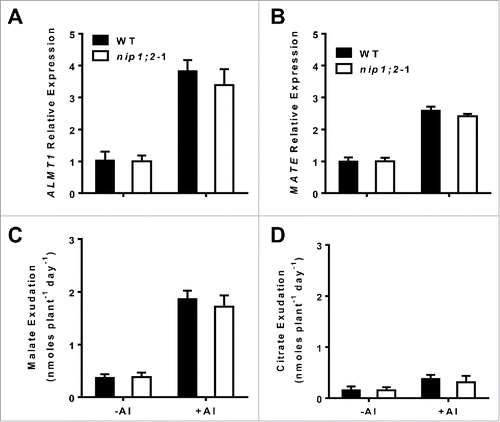
To investigate functional relationships between OA releases and NIP1;2-mediated Al transport, patterns of ALMT1 and MATE mRNA expression and root malate and citrate exudation were analyzed in the wild-type (WT) and nip1;2 mutant backgrounds (). qRT-PCR analyses indicated that in WT, the transcriptional expression of both ALMT1 and MATE was strongly induced by 24 h Al treatment (). Al treatment also led to releases of a large amount of malate and a smaller amount of citrate from the root (). These results are consistent with the previously reported.Citation11,Citation12 Compared with in the WT, the transcriptional expression of ALMT1 and MATE () and the ALMT1-mediate malate and MATE-mediated citrate exudation () were slightly, but insignificantly, lower in the nip1;2 mutant background, indicating that the nip1;2 mutation does not significantly affect ALMT1 and MATE gene expression and Al-activated malate and citrate exudation. In our recent study, we have demonstrated that MATE is not involved in the processes of the coordinated function between ALMT1 and NIP1;2.21 Taken together, our results suggest that ALMT1 is functionally epistatic to NIP1;2, but the ALMT1 function is independent of NIP1;2.
Figure 2. Patterns of transcriptional expression of ALMT1 (A) and MATE (B) and Al-activated malate (C) and citrate (D) exudation from the root in the wild type and the nip1;2 mutant. Here, 7-d-old seedlings were exposed to a hydroponic solution containing 30 μM AlCl3. Root samples were collected at 0, 6 h for qRT-PCR analysis. The Arabidopsis Ubiquitin gene (At1g31340) was used as an internal gene expression control. For root organic acid exudation assays, seedlings were treated with 0 or 30 μM AlCl3 for 24 h, then root exudation samples were collected for measuring malate and citrate contents. Values are means ± SD of three biological replicates.
Abbreviations
| ALMT1 | = | aluminum-activated malate transporter 1 |
| Al-Mal | = | aluminum malate |
| AQP | = | aquaporin |
| KO | = | knockout |
| MATE | = | multidrug and toxic compound extrusion |
| Nrat1 | = | Nramp aluminum transporter 1 |
| NIP | = | nodulin 26-like intrinsic protein |
| OAs | = | organic acids |
| PM | = | plasma membrane |
| STOP1 | = | sensitive to proton rhizotoxicity 1 |
| WT | = | wild type |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Von Uexküll H, Mutert E. Global extent, development and economic impact of acid soils. Plant Soil. 1995;171:1–15. doi:10.1007/978-94-011-0221-6_1.
- Kochian L, Piñeros M, Liu J, Magalhaes J. Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annu Rev Plant Biol. 2015;66:571–98. doi:10.1146/annurev-arplant-043014-114822.
- Liu J, Piñeros MA, Kochian LV. The role of aluminum sensing and signaling in plant aluminum resistance. J Integr Plant Biol. 2014;56:221–30. doi:10.1111/jipb.12162.
- Wood S, Sebastian K, Scherr S. Pilot Analysis of Global Ecosystems: Agroecosystems. World Resources Institute, Washington, DC. 2000.
- Ma JF. Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. Int Rev Cytol. 2007;264:225–52. doi:10.1016/S0074-7696(07)64005-4.
- Delhaize E, Gruber BD, Ryan PR. The roles of organic anion permeases in aluminium resistance and mineral nutrition. FEBS Lett. 2007;581:2255–62. doi:10.1016/j.febslet.2007.03.057.
- Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H. A wheat gene encoding an aluminum‐activated malate transporter. Plant J. 2004;37:645–53. doi:10.1111/j.1365-313X.2003.01991.x.
- Hoekenga OA, Maron LG, Piñeros MA, Cançado GM, Shaff J, Kobayashi Y, Ryan PR, Dong B, Delhaize E, Sasaki T, et al. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc Natl Acad Sci U S A. 2006;103:9738–43. doi:10.1073/pnas.0602868103.
- Magalhaes JV, Liu J, Guimaraes CT, Lana UG, Alves VM, Wang Y-H, Schaffert RE, Hoekenga OA, Piñeros MA, Shaff JE, et al. A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nature Genet. 2007;39:1156–61. doi:10.1038/ng2074.
- Furukawa J, Yamaji N, Wang H, Mitani N, Murata Y, Sato K, Katsuhara M, Takeda K, Ma JF. An aluminum-activated citrate transporter in barley. Plant Cell Physiol. 2007;48:1081–91. doi:10.1093/pcp/pcm091.
- Liu J, Magalhaes JV, Shaff J, Kochian LV. Aluminum‐activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J. 2009;57:389–99. doi:10.1111/j.1365-313X.2008.03696.x
- Liu J, Luo X, Shaff J, Liang C, Jia X, Li Z, Magalhaes J, Kochian LV. A promoter‐swap strategy between the AtALMT and AtMATE genes increased Arabidopsis aluminum resistance and improved carbon‐use efficiency for aluminum resistance. Plant J. 2012;71:327–37. doi:10.1111/j.1365-313X.2012.04994.x.
- Maron LG, Guimarães CT, Kirst M, Albert PS, Birchler JA, Bradbury PJ, Buckler ES, Coluccio AE, Danilova TV, Kudrna D, et al. Proc Natl Acad Sci U S A. 2013;110:5241–6. doi:10.1073/pnas.1220766110.
- Sawaki Y, Iuchi S, Kobayashi Y, Kobayashi Y, Ikka T, Sakurai N, Fujita M, Shinozaki K, Shibata D, Kobayashi M, et al. STOP1 regulates multiple genes that protect Arabidopsis from proton and aluminum toxicities. Plant Physiol. 2009;150:281–94. doi:10.1104/pp.108.134700.
- Iuchi S, Koyama H, Iuchi A, Kobayashi Y, Kitabayashi S, Kobayashi Y, Ikka T, Hirayama T, Shinozaki K, Kobayashi M. Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proc Natl Acad Sci U S A. 2007;104:9900–5. doi:10.1073/pnas.0700117104.
- Jiang F, Wang T, Wang Y, Kochian LV, Chen F, Liu J. Identification and characterization of suppressor mutants of stop1. BMC Plant Biol. 2017;17:128. doi:10.1186/s12870-017-1079-2.
- Xia J, Yamaji N, Kasai T, Ma JF. Plasma membrane-localized transporter for aluminum in rice. Proc Natl Acad Sci U S A. 2010;107:18381–5. doi:10.1073/pnas.1004949107.
- Li J-Y, Liu J, Dong D, Jia X, McCouch SR, Kochian LV. Natural variation underlies alterations in Nramp aluminum transporter (NRAT1) expression and function that play a key role in rice aluminum tolerance. Proc Natl Acad Sci U S A. 2014;111:6503–8. doi:10.1073/pnas.1318975111.
- Horst WJ, Wang Y, Eticha D. The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Ann Bot. 2010;106:185–97. doi:10.1093/aob/mcq053.
- Zhou D, Yang Y, Zhang J, Jiang F, Craft E, Thannhauser TW, Kochian LV, Liu J. Quantitative iTRAQ proteomics revealed possible roles for antioxidant proteins in sorghum aluminum tolerance. Front Plant Sci. 2016;7:2043. doi:10.3389/fpls.2016.02043.
- Wang Y, Li R, Li D, Jia X, Zhou D, Li J, Lyi SM, Hou S, Huang Y, Kochian LV, et al. NIP1;2 is a plasma membrane-localized transporter mediating aluminum uptake, translocation, and tolerance in Arabidopsis. Proc Natl Acad Sci U S A. 2017;114:5047–52. doi:10.1073/pnas.1618557114.
