ABSTRACT
Putative protein O-fucosyltransferases (POFTs) represent a large family of Glycosyl Transferase family 65 domain-containing proteins in land plants, with at least 39 proposed members in the Arabidopsis thaliana genome alone. We recently identified a member of this family, AtOFT1 (At3g05320), in which loss-of-function mutants display impaired sexual reproduction that was linked to a defective male gamete. Specifically, oft1 mutant pollen tubes are ineffective at penetrating the stigma-style interface leading to a drastic reduction in seed set and a nearly 2000-fold reduction in pollen transmission. Our findings establish that AtOFT1 plays a critical role in pollen tube penetration through the stigma/style in Arabidopsis and further suggest an important role for protein O-glycosylation events that potentially influence pollen tube mechanical strength or the ability to respond to positional guidance cues during the process of tube growth and fertilization.
Angiosperm sexual reproduction requires cooperative interactions between the male and female tissues to facilitate a process known as double-fertilization, in which the male gamete (pollen grain) must adhere to the stigmatic papillae, hydrate to produce a pollen tube (PT), and directionally grow downward into the female pistil tissues, where the PT must locate an unfertilized ovule and deliver its two sperm cells.Citation1-Citation5 The pistil provides guidance cues to lead the PT along its path to the distant ovule and supplies essential nutrients that sustain rapid PT growth through the pistil tissues.Citation6-Citation10 Additionally, the PT must circumvent numerous mechanical barriers by physically penetrating through cells and tissues, such as the papillar cell cuticle, stigma, style, and the micropyle.Citation3-Citation5 Recently, we characterized loss-of-function mutations in a putative protein O-fucosyltransferase (At3g05320; AtOFT1) from Arabidopsis thaliana. These mutants displayed a near-sterile phenotype, with an approximately 10-fold reduction in seed set.Citation11 This physiological defect suggested a critical role for AtOFT1 in sexual reproduction.
While manual outcrosses with wild-type Col-0 pollen on oft1 stigmas did not exhibit segregation distortion for T-DNA transmission efficiency, when oft1± pollen was used to fertilize Col-0 stigmas, only a single transmission event of the T-DNA allele could be detected out of 1,872 progeny, which strongly suggested that the impaired fertility of oft1 plants was due to a defective male gamete. Mutant oft1 PTs displayed no overt morphological or growth defects compared to Col-0 during in vitro germination. However, after manual pollination and imaging of analine blue stained tubes growing into the pistils of a male sterile 1 (ms1) host, we observed that oft1 PTs were largely retained in the style 24 hours after pollination (HAP), while Col-0 PTs had emerged into the ovary cavity. Under semi-in vivo (SIV) conditions, oft1 PTs exhibited at least a 1-hour delay in exiting the transmitting tract (TT) of ms1 pistils compared to Col-0. After 8 hours, the number of mutant PTs exiting the style was still 3-fold lower that WT. These results indicate that the stigma and style pose a significant reproductive barrier for oft1 mutant pollen and suggest that AtOFT1 is important for facilitating PT penetration through these female tissues. We further tested this hypothesis through a stigma/style-decapitation assay in which the stigma and style were dissected from the pistils, and pollen grains were germinated on the cut surface and allowed to grow into the ovary. When this assay was performed using pollen from a heterozygous plant that was segregating wild-type and oft1 mutant pollen, a 29.1% transmission of the mutant allele was observed. This dramatic improvement compared to the 0.1% transmission observed when pollinating an intact pistil further supported the hypothesis that the stigma and style critically interfere with the reproductive success of oft1 pollen. However, the observation that there is still a reduced transmission efficiency (29% instead of an expected 50% transmission of the mutant allele) even when the stigma/style barrier is removed, suggests that oft1 pollen are still impaired in penetrating additional physical barriers or responding to chemoattractants, as discussed below.
Protein O-fucosyltransferases (POFTs) are relatively understudied enzymes in plant systems. The Arabidopsis genome contains 39 putative POFT-like genes,Citation12,Citation13 and emerging evidence suggests an important role for these enzymes in plant systems, yet characterization of the biochemical activity of even a single family member has remained elusive.Citation14,Citation15 The Carbohydrate Active Enzyme Database (CAZy) classifies POFTs as GT65-domain containing proteins, and in metazoan systems, the GT65 class of enzymes is best represented by the enzyme POFT1.Citation13 These enzymes utilize GDP-fucose to transfer L-fucose onto serine or threonine residues of target proteins typically within defined consensus sequences residing in Epidermal Growth Factor (EGF) or Thrombospondin Repeat (TSR) domains.Citation16,Citation17 These post-translational modifications impart critical regulatory functions to fundamental biological pathways, such as the Notch signaling pathway, where mono-O-fucosylation of the Notch extracellular domain is essential for potentiating Notch interactions with Delta, Serrate, and Jagged ligands.Citation18-Citation22 Phylogenetic analysis of the putative Arabidopsis POFT family, revealed that AtOFT1 shares close sequence homology to metazoan POFT1s. Moreover, the crystal structure of Caenorhabditis elegans POFT1 was recently determined, which defined specific amino acid residues that participate in metazoan POFT1 catalysis,Citation17 and protein sequence alignment with AtOFT1 showed conservation in 4 of these 5 critical residues. We mutagenized these residues in AtOFT1 and assessed each mutant’s ability to complement the seed set phenotype of the oft1 background. Each of these conserved residues was essential for AtOFT1 in vivo function. Importantly, AtOFT1 H54 aligned to C. elegans POFT1 N43. When AtOFT1 H54N was retransformed into the oft1 mutant background, seed set was restored to that of wildtype. Overall, these results indicate that catalytically important residues for metazoan POFT1s are also functionally important in AtOFT1, supporting a working model that AtOFT1 might have a similar catalytic mechanism to metazoan POFTs.
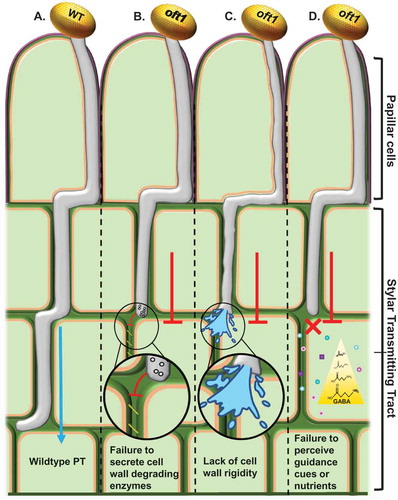
Among the Arabidopsis putative POFT family, 7 members have been previously phenotypically characterized, implicating these enzymes in maintaining proper cell wall architecture and/or cell adhesion.Citation14,Citation15,Citation23–Citation26 The central function of cell adhesion underlying the PT’s journey through the female tissues has long been established,Citation27,Citation28 and although AtOFT1’s particular role in this process remains unclear, we propose three hypotheses for the male-specific reproductive deficiency of oft1 pollen (). First, we postulate that AtOFT1 could help facilitate degradation of the female tissue cell wall by post-translationally activating enzymes involved in this process or potentiating their secretion (). Cell wall loosening molecules involved in fertilization play a role in PT invasion of female tissues in other species, such as Nicotiana tabacum and Brassica napus, suggesting that a similar degradative mechanism may be required in Arabidopsis.Citation3,Citation29–Citation32 Thus, oft1 PTs may fail to penetrate through the stigma and style due to their inability to degrade the pistil cell wall ECM. Second, AtOFT1 could conceivably participate in the synthesis of an unidentified pollen cell-wall component that facilitates PT penetration through the stigma/style (). Although oft1 pollen were indistinguishable from wild-type pollen in vitro, it is reasonable to speculate that a currently undefined PT cell wall rigidifying component is necessary to forcibly invade through the pistil tissues. Finally, AtOFT1 may post-translationally modify and potentiate a receptor that is necessary for responses to positional guidance cues within the stigma/style and TT (). Supporting this hypothesis is the similar defective penetration behavior observed in Arabidopsis VACUOLAR SORTING PROTEIN41 (VPS41) mutants, in which vsp41 PTs are proposed to be unable to recycle guidance cue receptors, resulting in arrested PT growth in the styleCitation33. Furthermore, PTs are known to become “capacitated” during invasion through the style by undefined mechanisms that alter the PT transcriptome, induce rapid polarized tip growth, and facilitate PT capacity to locate ovulesCitation34,Citation35. Impairing this process may additionally explain the “unmotivated” penetration behavior of oft1 PTs.
Figure 1. Potential models for AtOFT1 behavior during pollen tube penetration.
Illustration of proposed mechanisms for the oft1 mutant pollen tube penetration defect. A, Col-0 (WT) PT rapidly traversing the transmitting tract towards the ovary, while oft1 mutant PTs (B-D) exhibit slower penetration rates through these tissues, which most often results in arrested growth. B, The oft1 PT may fail to secrete or activate female tissue cell wall degrading enzymes, thus making passage through these tissues arduous. C, oft1 PT potentially lacks a cell wall rigidifying component that aids in penetrating though the female tissue layers during fertilization. D, oft1 PT potentially lacks a functional receptor that is necessary for the PT to respond to positional guidance cues within the stylar transmitting tract (TT) or to metabolize the nutrients supplied by the TT ECM. Borders around cells are cuticle (purple), cell wall (green), and plasma membrane (orange). The location of the papillar cells and the stylar transmitting tract are indicated.
Acknowledgments
This work was supported in part by the National Science Foundation EAGER Award to ISW (Award number IOS 1449068), a National Science Foundation Award to JFH (IOS 1056774) and a National Science Foundation Graduate Research Fellowship Award to DKS.
Additional information
Funding
References
- Palanivelu R, Johnson MA. Functional genomics of pollen tube-pistil interactions in Arabidopsis. Biochem Soc Trans. 2010;38:593–597. doi:10.1042/BST0380593.
- Beale KM, Johnson MA. Speed dating, rejection, and finding the perfect mate: advice from flowering plants. Curr Opin Plant Biol. 2013;16:590–597. doi:10.1016/j.pbi.2013.08.005.
- Elleman CJ, Franklin-Tong V, Dickinson HG. Pollination in species with dry stigmas: the nature of the early stigmatic response and the pathway taken by pollen tubes. New Phytol. 1992;121:413–424. doi:10.1111/nph.1992.121.issue-3.
- Jiang L, Yang SL, Xie LF, Puah CS, Zhang XQ, Yang WC, Sundaresan V, Ye D. VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. THE PLANT CELL ONLINE. 2005;17:584–596. doi:10.1105/tpc.104.027631.
- Sessions RA, Zambryski PC. Arabidopsis gynoecium structure in the wild and in ettin mutants. Development. 1995;121:1519–1532.
- Palanivelu R, Brass L, Edlund AF, Preuss D. Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell. 2003;114:47–59.
- Okuda S, Tsutsui H, Shiina K, Sprunck S, Takeuchi H, Yui R, Kasahara RD, Hamamura Y, Mizukami A, Susaki D. et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature. 2009;458:357–361. doi:10.1038/nature07882.
- Takeuchi H, Higashiyama T. The human brain online: an open resource for advancing brain research. PLoS Biol. 2012;10:e1001449. doi:10.1371/journal.pbio.1001449.
- Takeuchi H, Higashiyama T. Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature. 2016;531:245–248. doi:10.1038/nature17413.
- Mizukami AG, Inatsugi R, Jiao J, Kotake T, Kuwata K, Ootani K, Okuda S, Sankaranarayanan S, Sato Y, Maruyama D. et al. The AMOR arabinogalactan sugar chain induces pollen-tube competency to respond to ovular guidance. Curr Biol. 2016;26:1091–1097. doi:10.1016/j.cub.2016.02.040.
- Smith DK, Jones DM, Lau JBR, Cruz ER, Brown E, Harper JF, Wallace IS. A putative protein O-fucosyltransferase facilitates pollen tube penetration through the stigma-style interface. Plant Physiology. 2018;17:2804–2818. doi:10.1104/pp.17.01577.
- Hansen S, Bettler E, Wimmerova M, Imberty A, Lerouxel O, Breton C. Combination of several bioinformatics approaches for the identification of new putative glycosyltransferases in arabidopsis. Journal of Proteome Research. 2009;8:743–753. doi:10.1021/pr800808m.
- Hansen S, Harholt J, Oikawa A, Scheller H. Plant glycosyltransferases beyond CAZy: a perspective on DUF families. Frontiers in Plant Science. 2012;3:59. doi:10.3389/fpls.2012.00154.
- Neumetzler L, Humphrey T, Lumba S, Snyder S, Yeats TH, Usadel B, Vasilevski A, Patel J, Rose JK, Persson S. et al. The FRIABLE1 gene product affects cell adhesion in Arabidopsis. PLoS ONE. 2012;7:e42914. doi:10.1371/journal.pone.0042914.
- Verger S, Chabout S, Gineau E, Mouille G. Cell adhesion in plants is under the control of putative O-fucosyltransferases. Development. 2016;143:2536–2540. doi:10.1242/dev.132308.
- Wang Y, Shao L, Shi S, Harris RJ, Spellman MW, Stanley P, Haltiwanger RS. Modification of epidermal growth factor-like repeats with O-fucose: molecular cloning and expression of a novel GDP-fucose protein O-fucosyltransferase. J Biol Chem. 2001;276:40338–40345. doi:10.1074/jbc.M107849200.
- Lira-Navarrete E, Valero-González J, Villanueva R, Martínez-Júlvez M, Tejero T, Merino P, Panjikar S, Hurtado-Guerrero R. Structural insights into the mechanism of protein O-fucosylation. PLoS ONE. 2011;6:e25365. doi:10.1371/journal.pone.0025365.
- Okajima T, Xu A, Irvine KD. Modulation of notch-ligand binding by protein O-fucosyltransferase 1 and fringe. J Biol Chem. 2003;278:42340–42345. doi:10.1074/jbc.M308687200.
- Sasamura T, Sasaki N, Miyashita F, Nakao S, Ishikawa HO, Ito M, Kitagawa M, Harigaya K, Spana E, Bilder D. et al. Neurotic, a novel maternal neurogenic gene, encodes an O-fucosyltransferase that is essential for Notch-Delta interactions. Development. 2003;130:4785–4795.
- Shi S, Stanley P. Protein O-fucosyltransferase 1 is an essential component of Notch signaling pathways. Proc Natl Acad Sci USA. 2003;100:5234–5239. doi:10.1073/pnas.0831126100.
- Rampal R, Li AS, Moloney DJ, Georgiou SA, Luther KB, Nita-Lazar A, Haltiwanger RS. Lunatic fringe, manic fringe, and radical fringe recognize similar specificity determinants in O-fucosylated epidermal growth factor-like repeats. J Biol Chem. 2005;280:42454–42463. doi:10.1074/jbc.M509552200.
- Stahl M, Uemura K, Ge C, Shi S, Tashima Y, Stanley P. Roles of Pofut1 and O-fucose in mammalian Notch signaling. J Biol Chem. 2008;283:13638–13651. doi:10.1074/jbc.M802027200.
- Nikolovski N, Rubtsov D, Segura MP, Miles GP, Stevens TJ, Dunkley TPJ, Munro S, Lilley KS, Dupree P. Putative glycosyltransferases and other plant golgi apparatus proteins are revealed by LOPIT proteomics. Plant Physiology. 2012;160:1037–1051. doi:10.1104/pp.112.204263.
- Wang Y, Mortimer JC, Davis J, Dupree P, Keegstra K. Identification of an additional protein involved in mannan biosynthesis. Plant J. 2013;73:105–117. doi:10.1111/tpj.12019.
- Stonebloom S, Ebert B, Xiong G, Pattathil S, Birdseye D, Lao J, Pauly M, Hahn MG, Heazlewood JL, Scheller HV. A DUF-246 family glycosyltransferase-like gene affects male fertility and the biosynthesis of pectic arabinogalactans. BMC Plant Biology. 2016;16:90.doi:10.1186/s12870-016-0780-x.
- Won SK, Lee YJ, Lee HY, Heo YK, Cho M, Cho HT. cis-element- and transcriptome-based screening of root hair-specific genes and their functional characterization in arabidopsis. Plant Physiology. 2009;150:1459–1473. doi:10.1104/pp.109.140905.
- Lennon KA, Roy S, Hepler PK, Lord EM. The structure of the transmitting tissue of Arabidopsis thaliana (L.) and the path of pollen tube growth. And the Path of Pollen Tube Growth. Sexual Plant Reproduction. 1998;11:49–59. doi:10.1007/s004970050120.
- Chae K, Lord EM. Pollen tube growth and guidance: roles of small, secreted proteins. Ann Bot. 2011;108:627–636. doi:10.1093/aob/mcr015.
- Cosgrove DJ, Bedinger P, Durachko DM. Group I allergens of grass pollen as cell wall-loosening agents. Proc. Natl. Acad. Sci. USA. 1997;94:6559–6564. doi:10.1073/pnas.94.12.6559.
- Pezzotti M, Feron R, Mariani C. Pollination modulates expression of the PPAL gene, a pistil-specific beta-expansin. Plant Molecular Biology. 2002;49:187–197. doi:10.1023/A:1014962923278.
- Dearnaley JDW, Daggard GA. Expression of a polygalacturonase enzyme in germinating pollen of Brassica napus. Sex. Plant Reproduction. 2001;13:265–271. doi:10.1007/s004970000062.
- Sanchez AM, Bosch M, Bots M, Nieuwland J, Feron R, Mariani C. Pistil factors controlling pollination. The Plant Cell. 2004;16:S98–S106. doi:10.1105/tpc.017806.
- Hao L, Liu J, Zhong S, Gu H, Qu LJ. AtVPS41-mediated endocytic pathway is essential for pollen tube-stigma interaction in Arabidopsis. Proc Natl Acad Sci USA. 2016;113:6307–6312. doi:10.1073/pnas.1602757113.
- Palanivelu R, Preuss D. Distinct short-range ovule signals attract or repel Arabidopsis thaliana pollen tubes in vitro. BMC Plant Biology. 2006;6:7. doi:10.1186/1471-2229-6-24.
- Geitmann A, Palanivelu R. Fertilization requires communication: signal generation and perception during pollen tube guidance. Floriculture and Ornamental Biotechnology. 2007;1:77–89.
