ABSTRACT
Pollination drop (PD) is a characteristic feature of major wind-pollinated gymnosperms and plays a vital role during the course of pollination, however, the composition and proteomic profile of PDs in Ginkgo biloba remain unclear. Through inductively coupled plasma mass spectrometry, we detected mineral elements in PDs, including calcium (Ca), sulfur (S), magnesium, boron, and potassium (K), among which S, Ca, and K were found at high levels. The total sugar concentration was approximately 5.908 mg/mL, which accounted for approximately 5.9% (mass ratio) of the PD. The sugars primarily consisted of fructose, glucose, and sucrose, of which the glucose level was highest, accounting for 57.6%, followed by fructose (37.1%) and sucrose (5.3%). We also used FTIR to validate the presence of sugars and proteins in PDs. Further proteomic analysis revealed that the PD contained calmodulin, α-L-arabinofuranosidase, β-D-xylosidase, superoxide dismutase, α-L-arabinosidase, glutathione S-transferase, histones, glycine-rich family protein, methionine synthase, and arabinogalactan, suggesting that proteins present in PDs of G. biloba play a critical role in the defense against external bacteria and facilitate germination and growth of the pollen tube. Our results suggest that PDs are not merely a medium to receive and transport pollen but may also play a more complex biological role in pollination and fertilization.
Introduction
In wind-pollinated gymnosperms, pollen grains are transported by air to the ovule. The ovules of most gymnosperms produce secretions during the pollination period, which are extruded from the micropyle.Citation1 This ovular secretion, known as the pollination drop (PD), is regarded as the pollen-landing site in most gymnosperms.Citation2 PDs increase the surface area for pollen–ovule contact, maintaining pollen vitality and transporting pollen into the ovule. Pollen in the air is captured by PDs outside the micropyle, immersed into the PD, and transported into the micropyle canal mainly via PD withdrawal.Citation3
The interaction between pollen and PDs is the first step of post-pollination, and thus the composition of the PD could play an important role in this process. Early studies showed that PDs are composed of a variety of relatively simple water-soluble compounds. However, increasing evidence has suggested that a number of chemical compounds are present in the PDs of gymnosperms. The main constituents include various carbohydrates, such as fructose, glucose, and sucrose, although the sugar concentration in PDs has been shown to vary significantly among taxa. The predominant sugars (sucrose, glucose, and fructose) range from 12.5 mg/mL in PinusCitation4 to 250 mg/mL in Ephedra.Citation5 Moreover, in angiosperm nectar, sucrose is the most common sugar and is present in almost 90% of 765 species.Citation6 However, fructose is the predominant sugar in the PDs of most gymnosperms, including Pinus mugo, Thuja orientalis, Taxus baccata, and Cephalotaxus drupacea,Citation7,Citation8 and primarily facilitates pollen tube development.Citation1 Some sugar aldehydes have also been identified in PDs. Additionally, abundant uronic acids have been shown to be released following the degeneration of nucellus cells in PDs of C. drupacea.Citation8 Certain sugar aldehydes, detected in PDs of Thuja and Taxus, likely inhibit some pathogens.Citation9 Importantly, similar to the composition of angiosperm nectar, amino acids and proteins also exist in the PDs of gymnosperms.Citation10 Earlier studies have focused on conifer species; e.g., in Thuja, PDs contain serine, acetic acid, alanine, and glutamic acid salt.Citation11 Recent research has also identified serine, glutamic acid, glycine, histidine, alanine, and proline, which have been shown to be abundant in the ovular secretions of numerous gymnosperms, including Ephedra minuta, Zamia furfuracea, Cephalotaxus koreana, Chamaecyparis lawsoniana, and T. baccata.Citation12
With the application of proteomics technologies, functional proteins have been detected in the PDs of several gymnosperms.Citation13–Citation15 The proteins detected in PDs can be divided into five categories: (1) those involved in the modification of sugars, e.g., xylosidase and galactosidase,Citation13 (2) those serving a defense-like function, e.g., glucan-β-1,3-glucosidase, chitinase, and thaumatin-like protein,Citation14–Citation16 (3) those associated with the freeze-proofing function,Citation17 (4) those associated with plant cell metabolism, e.g., peroxidase,Citation18 and (5) those capable of accelerating pollen tube growth, e.g., aspartyl protease and serine carboxypeptidase.Citation13 However, most studies investigating the proteins present in PDs have focused on conifers, whereas detailed analyses on non-conifer PDs in gymnosperms are lacking.
Ginkgo biloba L., one of the oldest lineages of gymnosperms, is dioecious with wind pollination. During the pollination stage, PDs are secreted from the surface of the micropyle at the top of the ovule, and mature pollen sheds from microsporangia floating in the air. Once pollen is trapped by PDs, it immediately hydrates, triggering PD withdrawal, and is subsequently carried into the ovule, where it germinates and undergoes fertilization.Citation19,Citation20 Although several lines of evidence have indicated that PDs play a vital role during pollination in G. biloba, far less is known about individual components and the functions in the secretions. In the present study, we used FTIR, ICP-MS and HPLC-MS to determine the composition of PDs, and detected sugars, mineral elements, and proteins in G. biloba PDs. Our results provide insight into the function of PDs in the interaction with pollen during pollination and fertilization.
Results
Mineral elements present in PDs
Mineral elements are important for the synthesis and metabolism of a variety of enzymes. We used ICP-MS to analyze the mineral elements in PDs and detected high levels of sulfur (S), calcium (Ca), potassium (K), and sodium (Na) in PDs (). The element with the highest concentration was S (3.498 ppm), although Ca and K were also present at high levels (0.336 and 0.116 ppm, respectively). Magnesium (Mg), boron (B), aluminum (Al), silicon (Si), zinc (Zn), and iron (Fe) were also detected in small amounts; the levels of Mg, B, and Zn were 0.013, 0.005, and 0.002 ppm, respectively ().
Table 1. Contents of different elements in the PDs of G. biloba.
Sugars present in PDs
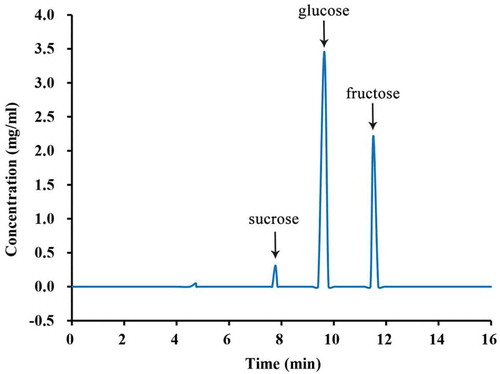
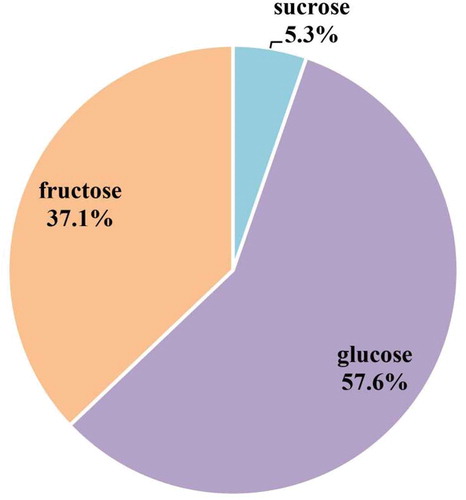
The composition and proportion of sugars in PDs are important for pollen germination, pollen tube growth, and fertilization. To investigate the types and levels of sugars in PDs, we measured soluble sugars using HPLC. We found that the soluble sugars in PDs of G. biloba consisted mainly of fructose, glucose, and sucrose, and the total content of these sugars was approximately 5.9% of the total PD (5.908 mg/mL). Glucose was present at the highest concentration (3.403 mg/mL), accounting for 57.6%, followed by fructose (2.193 mg/mL; 37.1%) and sucrose (0.312 mg/mL; 5.3%) ( and ). As shown in , the levels of the three sugar types in PDs varied significantly; the glucose level was higher than the fructose level, and sucrose was present at the lowest level.
FTIR analysis of PD components
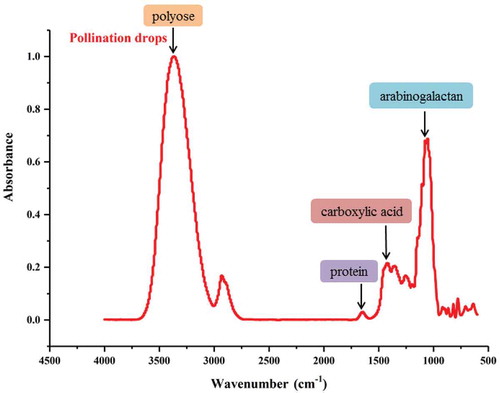
We used FTIR, which can be used to analyze the ingredients of intact samples quickly, to detect the components of PDs in G. biloba. shows that the PDs had a typical absorption spectrum. The major absorption peaks represented polysaccharide hydroxyl at 3400 cm−1, carboxylic acid at 1450 cm−1, the amino tensile bond of proteins at 1551 cm−1, and carbohydrates at 1200–900 cm−1. Moreover, the FTIR absorption spectrum revealed that arabinogalactan was a component of PDs, with an absorption peak at 1080 cm−1. These results indicated that G. biloba PDs contain polysaccharides, proteins, and carbohydrates.
Identification of proteins in PDs
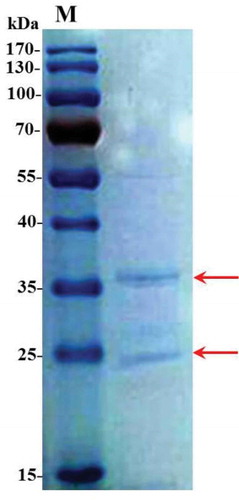
We firstly detected two distinct main bands at 25 kd and 36 kd in G. biloba PDs through 1-D SDS-PAGE (). Then the two main protein bands were excised from gels and were analyzed by Mass Spectrometer. We totally obtained 27 proteins in G. biloba PDs. These proteins were successfully annotated by searching against protein BLAST of the Paragon database. Through functional annotation, many were involved in 1) the sugar metabolism, including glyceraldehyde 3-phosphate dehydrogenase, α-L-arabinofuranosidase, β-D-xylosidase and α-L-arabinosidase; 2) protein synthesis, including methionine synthase, 40S ribosomal protein S3 and histone H4; 3) singal transduction, such as calmodulin; 4) catalytic reaction, such as glutathione S-transferase and methionine synthase. In addition, other proteins with more functions may also contribute to the pollination process of G. biloba ().
Table 2. Proteins identified by mass spectrometry in the PDs of G. biloba.
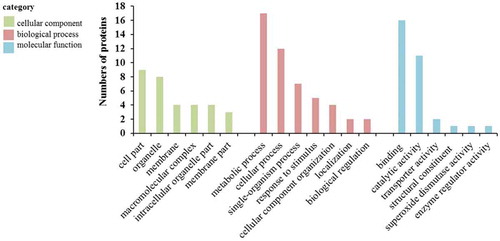
To better understand the biological functions of these identified proteins, GO annotation was performed, followed by GO functional classification (cellular component, biological process, and molecular function). In terms of cellular component, the two dominant GO terms were cell part and organelle, followed by membrane, macromolecular complex and intracellular organelle part. In biological process category of the identified proteins, the two largest groups of proteins were corresponded to metabolic process and cellular process subcategories. Additionally, there were also some proteins involved in single-organism process, response to stimulus, cellular component organization and localization. For molecular function category, binding and catalytic activity were in the top two terms. Transporter activity, structural constituent, superoxide dismutase activity and enzyme regulator activity were also found, but in a low component (). In short, most of the identified proteins were related to binding, catalytic activity, metabolic and cellular processes, and response to stimulus.
Discussion
The majority of gymnosperms are pollinated by wind, and the PD is the most common type of ovule secretion used to capture pollen, playing a critical role in reproduction. Consequently, the chemical composition and function of PDs have long been a focus of gymnosperm reproduction research. Mineral elements have different effects on the reproduction process, such as pollen hydration, germination, and growth.Citation21 In angiosperms, several studies have demonstrated that pollen germination together with the oriented growth of the pollen tube is closely associated with Ca.Citation22 Moreover, B can also stimulate pollen germination and pollen tube elongation by influencing the accumulation of callose, acidic pectin, phenolics and saturated esters in the pollen tube.Citation23 Moreover, Zn is capable of facilitating pollen germination, pollen tube elongation, and fertilization, which ultimately enhanced fertilization success. Applying Zn fertilizer can accelerate auxin synthesis and transport photosynthate to reproductive organs, which may supply sufficient inclusions for pollen, avoiding pollen abortion.Citation24 K ion also contributes to the growth of pollen tube, the influx of K+ mediated by ZmES4-activated KZM1 can trigger rapid plasma membrane depolarization, which induced the pollen tube tip burst.Citation25 Although mineral elements have important roles in reproduction, the ephemeral nature and small quantity of PDs have significantly limited PD collection and constituent analysis, especially with respect to the detection and analysis of mineral elements in gymnosperm PDs. In the present study, we used ICP-MS to comprehensively analyze the mineral elements of G. biloba PDs and detected Ca, B, Zn, and K in the PDs of G. biloba, with Ca and K present in the highest concentrations. According to their function, we inferred that these elements may play similar roles in pollen germination, growth, and elongation of the pollen tube. We also detected other elements, including S, Na, Mg, phosphorus, Si, and Al, in the PDs. Among the common elements, S was present in relatively higher concentrations. S is not only a critical component of amino acids and proteins but participates in electron transfer, substrate binding/activation, regulation of gene expression, and enzyme activity,Citation26 suggesting it might be involved in the synthesis and degradation of various components and substances whose metabolism is increased during the pollination period in PDs.
A large variety of animal pollinators rely on sugary secretions for their nutrition, and the sugary secretions primarily serve as a reward for pollinators in angiosperms.Citation27 However, the PDs of the majority of wind-pollinated gymnosperms also contain sugary secretions, even though PDs function as the pollen landing site and the transporting medium carrying pollen into the nucellus. Although their functions differ, both PDs and nectars contain fructose, glucose, and sucrose.Citation1,Citation28 However, the sugar contents differ significantly between nectar and PDs. Sucrose was frequently identified as the dominant sugar in angiosperm nectar, while this was not the case in gymnosperm PDs.Citation1,Citation6 Importantly, PDs possess a much lower concentration of sugars than does nectar and contain certain sugars, such as xylose, which are unattractive to pollinators.Citation29 Although PDs contain attractive sugars, such as sucrose, they were present in low concentrations. Thus, PDs are generally poor food sources for insect pollinators. Additionally, in gymnosperms PDs, the concentrations of different sugars have been shown to vary greatly from species to species. In Pinus nigra, PDs contain 1.25% total sugar, and fructose was the dominant sugar, followed by glucose and sucrose.Citation4 In terms of total sugar content, the PDs of C. drupacea contain 77% fructose.Citation8 By contrast, PDs of Picea engelmannii had a higher concentration of glucose (4.3%) than fructose (3.8%) and did not contain sucrose.Citation30 Our FTIR results demonstrated that PDs of G. biloba are composed of a variety of ingredients, sugars, carbohydrates, and proteins. HPLC analysis further determined that total soluble sugar was present at approximately 5.9%, which was a higher content than that of other anemophilous gymnosperms (1–2%),Citation4 but lower than G. biloba reported by Nepi et al. (34.7 ± 8.1%).Citation12 The reason for the low sugar content in our study may be because of the collection of PDs in relatively humid conditions. In most anemophilous gymnosperm ovular secretions, fructose tends to be the dominant sugar;Citation1 however, in G. biloba PDs, the glucose content was higher than that of fructose, and the sucrose concentration was the lowest. Together with our previous study on G. biloba PDs,Citation20 our results suggest that the different levels of the three sugars might play a role in optimizing the selection of conspecific pollen by creating a suitable chemical medium, which could be beneficial for pollen germination, growth, and the resulting fertilization process.
The existence of proteins in the PDs of many gymnosperms has been reported recently, and the identified proteins strongly indicate that the biological roles of PDs might be more complex than simply the receipt and transportation of pollen during pollination.Citation31–Citation33 The number of proteins varies from species to species in gymnosperms PDs. For instance, the number of characterized proteins in degradome and secretome of Ephedra species PDs ranged from 6 to 20,Citation32 which were less than that of G. biloba. However, a total of 30 and 32 proteins were identified in C. sinensis and C. koreana PDs, respectively.Citation33
More importantly, there were some proteins in common among many gymnosperms PDs, which played similar function during the period of pollination and fertilization. Previous studies have found that the presence of enzymes with the ability to cleave xyloglucan support chains, such as xylosidases and galactosidases, resulted in cell wall loosening, which would be beneficial for pollen germination and growth of the pollen tube.Citation34,Citation35 Peroxidase has been identified in Douglas fir ovular secretions, which might be associated with selective function by producing reactive oxygen intermediates that have been shown to cleave pollen cell walls.Citation13 Some studies have also demonstrated that α-L-arabinofuranosidase can assist other hemicellulases in degrading hemicellulose.Citation36,Citation37 Arabinogalactan is a glycoprotein that exists widely in higher plants. In angiosperms, when pollination occurs, a large amount of arabinogalactan is secreted into the stigma papilla and the style-transmitting tissue, possibly playing a role as biochemical support or directional clues for the pollen tube to reach its target.Citation38 Arabinogalactan might be involved in the interaction between pollen and stigma or that between pollen and pollen. It also functions in the exclusion of heterogeneous pollen and the protection of pollen against infection from external pathogens, and may play a guiding role in the growth of the pollen tube in gymnosperms.Citation39 In addition, similar to dextran 1, 3-β glucosidase and thaumatin-like proteins, superoxide dismutase plays a role in fungal defense in the gymnosperm secretions of various species.Citation32 Glutathione S-transferase can catalyze covalent binding of reduced glutathione to hydrophobic and electrophilic substrates, subsequently forming a conjugate that degrades endogenous and extraneous harmful substances.Citation40 To date, the predicted functions of PD proteins range from pollen selection and development to pathogen defenses. Based on the FTIR and MS analyses in this study, many of the proteins detected in the PDs of G. biloba can be classified into four categories: (1) proteins associated with sugar modification, e.g., α-L-arabinofuranosidase and xylosidase, (2) proteins associated with defense, e.g., superoxide dismutase and glutathione S-transferase, (3) proteins associated with metabolism, e.g., glycine-rich family protein and methionine synthase, and (4) proteins that facilitate growth of the pollen tube, e.g., calmodulin and arabinogalactan. Our results suggest that the PD proteins of G. biloba participate in different aspects of pollen development, such as pollen germination, elongation of the pollen tube, and defense against both fungi and bacteria. Moreover, combined with GO annotation, the best-represented GO terms for three categories were the following: most proteins participating in metabolic processes, such as alpha-L-arabinofuranosidase, beta-D-xylosidase 4 and calmodulin; some proteins involved in the response to stimulus, including predicted isoflavone reductase homolog IRL-like, putative receptor-like GPI-anchored protein 2, etc.; and some proteins related to binding and catalytic activity, for example, C3HL domain class transcription factor, histone H4, small rubber particle protein, nucleosome positioning protein, methionine synthase and glyceraldehyde-3-phosphate dehydrogenase. These results further indicate that abundant metabolic processes such as enzyme catalytic reactions, carbohydrate metabolism and gamete recognition processes occurred in PDs. Taken together, we believe that the PDs of G. biloba play several roles, including supplying a suitable medium for pollen recognition, pollen tube germination, and growth.
Materials and methods
Plant material
PD samples from G. biloba were collected at the Ginkgo Experimental Station at Yangzhou University, Yangzhou, China (32°20ʹN, 119°30ʹE). Healthy female G. biloba trees with sufficient ovules were collected from approximately 30 plants of similar age and height during the pollination period in early April 2014 and 2015. Branches with ovules that had just secreted a PD were collected and kept in the laboratory. Short shoots with 3–5 ovules were cut with secateurs and inserted into a sponge that was immersed in water (). While we collected approximately 6000 ovules, we obtained only a small number of PDs; thus, some experiments were difficult to repeat. PDs were removed with a pipette into a 1.5 mL sterile storage tube after reaching the maximum volume and stored at −20°C until analysis.
Determination of mineral elements in PDs
ICP-MS has several advantages, including high accuracy, a wide dynamic linear range, simultaneous determination of multiple elements, and rapid detection and analysis. PDs can be tested directly without any special pretreatment steps. Prior to analysis, the PD samples stored at −20°C were thawed on ice, and 5 mL centrifuge tubes were soaked with concentrated nitric acid for at least 24 h to eliminate interference caused by other foreign ions. PDs (400 μL) were diluted with ultrapure water (1: 10) and sent to Yangzhou University Testing Center for analysis. Analysis of mineral elements was performed directly by ICP-MS (Elan DRC-e, PerkinElmer, Waltham, MA, USA). Samples were compared with ultrapure water as a control, and the results were expressed in ppm.
Detection of soluble sugars in PDs
Sugar content analysis was conducted by high performance liquid chromatography (HPLC). PDs (100 μL) were filtered through a 0.45 µm microporous membrane, and a 10 µL sample of the filtered PDs was injected into a Sugar-Pak I column (Carbo Sep CHO-620 CA Carbohydrate, Transgenomic, Inc., Omaha, NE, USA) maintained at 80°C. Ultrapure water after degassing was used as the mobile phase for elution at a flow rate of 0.5 mL/min. The elution peaks were detected using the LC-20AT and RID-10A detector, and Empower 3 software was used to control the systematic operation and process the results. The standard samples (glucose, fructose, sucrose, and sorbitol) were all chromatographically pure standard products (solid).
FTIR detection in PDs
The PDs were dripped onto the BaF2 wafer (barium fluoride window) and desiccated for 30 min at 50°C. FTIR spectra were recorded using the PerkinElmer Cetus MAGNA 750 FTIR spectrograph (Nicolet Corp., Tokyo, Japan) equipped with an MCT detector and microscope, with a spectral resolution of 16 cm−1, measurement range of 2000–800 cm−1, and the scan times were 128. Data were analyzed in Excel using the appropriate absorption wave number (wave numbers cm−1), and the y-axis represented the relative absorbance (absorbance).
Extraction and detection of total protein in PDs
PDs (200 μL) were injected into the vacuum freeze dryer for approximately 5 h to concentrate the samples, and 20 μL were removed and mixed with 2 μL 2 × sample buffer. Protein denaturation was then conducted at 99°C for 10 min, 20 μL 2 × sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis loading buffer were added, and the mixture was immersed in boiling water for 3–5 min to fully denature the proteins. The protein concentration was determined by spectrophotometry. A mixture of 1 μL protein extract and 99 μL HPLC-H2O was used as a control. For detection, 99 μL HPLC-H2O were added to 1 μL each sample, with three repeats performed per sample, followed by the addition of 3 mL Coomassie Blue staining solution and incubation for 3 min. The absorbance of the sample was detected at 595 nm, and after computing the mean absorbance values, the protein concentration was calculated as follows: protein concentration = (548.58 × absorbance value – 0.2861)/10.Citation41
Proteins of PDs separated by 1-D gel electrophoresis
Prior to electrophoresis, 10 mL of 12% polyacrylamide resolving gel and 5 mL of 4% polyacrylamide stacking gel were prepared, following Laemmli.Citation42 A total of 20 μL protein sample was loaded into 12% self-poured SDS-PAGE gel, and separated using electrophoresis. 5–10 μL of molecular weight protein markers (14.4–200 kDa) were used as standards, and proteins were visualized by staining with Coomassie Brilliant Blue (CBB) G-250.
Mass spectrometry (MS) of PDs
Prior to MS analysis, protein samples (50 μg) were supplemented with 0.5 M triethylammonium bicarbonate (TEAB) to 50 μL. The samples were reduced for 1 h at 60°C and alkylated for 1 h in the dark at room temperature. SDS in the reduced and alkylated protein solutions was eliminated by the addition of 0.05 M TEAB (pH 8.5) solution, and 200 μL 0.5 M TEAB (pH 8.5) solution and 10 μL μg/μL trypsin were added for enzymolysis overnight at 37°C. Then, the samples were centrifuged at 12,000 × g for 20 min, 200 μL 0.05 M TEAB (pH 8.5) solution were added to the digested peptide solution, and the solution was collected after centrifugation and desiccated by vacuum freezing.
MS analysis was performed using a Na upgrade inverting chromatography–TripleTOF 5600 system, NanoLC system (NanoLC-2D Ultra, Eksigent Technologies, Dublin, CA, USA) and TripleTOF 5600+ mass spectrometer (AB Sciex, Framingham, MA, USA). Lyophilized samples were dissolved in 100 μL solvent A (0.1% formic acid in 2% acetonitrile), centrifuged at 12,000 × g for 20 min, and the liquid supernatant was desalted using a Ziptip prior to loading. The injected sample volume was 10 μL, and the sandwich method was performed to load the samples at a flow rate of 2 μL/min. The samples were eluted with salt in a trap column (100 μm × 20 mm) and introduced into the analytical column (75 μm × 150 mm). Two columns were packed with Magic C18-AQ 5 μm 200 Å phase packing material (Michrom Bioresources, Auburn, CA, USA). Peptides were eluted using a 5–30% concentration gradient of solvent B (0.1% formic acid in 98% acetonitrile) for 35 min at a flow rate of 0.3 μL/min. The identification and quantification of proteins were performed using ProteinPilot 4.5 software (AB Sciex). Peptide amino acid sequences with a high global false discovery rate (>90%) were submitted to a protein BLAST search of the Paragon database.
Analysis and functional annotation of proteins
Detected proteins were analyzed using ProteinPilot software, and the Uniprot database (http://www.uniprot.org/) and Blast2go (https://www.blast2go.com/) were used to conduct gene ontology (GO) annotation and functional classification of proteins.
Abbreviations
| PD: | = | pollination drop |
| G. biloba: | = | Ginkgo biloba |
| C. drupacea: | = | Cephalotaxus drupacea |
| T. baccata: | = | Taxus baccata |
| C. sinensis: | = | Cephalotaxus sinensis |
| C. koreana: | = | Cephalotaxus koreana |
| FTIR: | = | Fourier transform infrared spectroscopy |
| ICP-MS: | = | inductively coupled plasma mass spectrometry; |
| HPLC: | = | high performance liquid chromatography |
| SDS-PAGE: | = | sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| GO: | = | gene ontology |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported financially by Natural Science Foundation of China (No. 31670181; No. 31670695) and Natural Science Foundation of Jiangsu Province grant number (No. BK20161332).
Additional information
Funding
References
- Gelbart G, von Aderkas P. Ovular secretions as part of pollination mechanisms in conifers. Ann For Sci. 2002;59(4):345–357. doi:10.1051/forest:2002011.
- Labandeira CC, Kvaček J, Mostovski MB. Pollination drops, pollen, and insect pollination of Mesozoic gymnosperms. Taxon. 2007;56(3):663–695. doi:10.2307/25065853.
- Anderson ED, Owens JN. Microsporogenesis, pollination, pollen germination and male gametophyte development in Taxus brevifolia. Ann Bot. 2000;86(5):1033–1042. doi:10.1006/anbo.2000.1274.
- Mcwilliam JR. The role of the micropyle in the pollination of Pinus. Bot Gaz. 1958;120(2):109–117. doi:10.1086/336010.
- Ziegler H. Über die zusammensetzung des “Bestäubungstropfens” und mechanismus seiner secretion. Planta. 1959;52(6):587–599. doi:10.1007/BF01914757.
- Baker HG, Baker I. A brief historical review of the chemistry of floral nectar. In: Bentley B, Elias TS, editors. The biology of nectaries. New York (USA): Columbia University Press; 1983. p. 126–152.
- Nygaard P. Utilization of exogenous carbohydrates for tube growth and starch synthesis in Pine pollen suspension cultures. Physiol Plant. 1977;39(3):206–210. doi:10.1111/j.1399-3054.1977.tb04037.x.
- Seridi-Benkaddour R, Chesnoy L. Secretion and composition of the pollination drop of Cephalotaxus drupacea (Gymnosperm, Cephalotaxaceae). In: Cresti M, Gori P, Pacini E, editors. Sexual Reproduction in Higher Plants. Berlin (Germany): Springer Verlag; 1988. p. 345–350.
- Barner H, Christiansen H. The formation of pollen, the pollination mechanism, and the determination of the most favourable time for controlled pollination in Pseudotsuga menziesii. Silvae Genet. 1962;11(4):89–102. https://eurekamag.com/research/014/248/014248517.php.
- Nepi M, Soligo C, Nocentini D, Abate M, Guarnieri M, Cai G, Bini L, Puglia M, Bianchi L, Pacini E. Amino acids and protein profile in floral nectar: much more than a simple reward. Flora. 2012;207(7):475–481. doi:10.1016/j.flora.2012.06.002.
- Chesnoy L. Les sécrétions dans la pollinisation des gymnospermes. Acta Bot Gall. 1993;140(2):145–156. doi:10.1080/12538078.1993.10515579.
- Nepi M, Little S, Guarnieri M, Nocentini D, Prior N, Gill J, Tomlinson PB, Ickert-Bond SM, Pirone C, Pacini E, et al. Phylogenetic and functional signals in gymnosperm ovular secretions. Ann Bot. 2017;120(6):923–936. doi10.1093/aob/mcx103.
- Poulis BAD, Sjb O, Haddow JD, von Aderkas P. Identification of proteins present in the Douglas fir ovular secretion: an insight into conifer pollen selection and development. Int J Plant Sci. 2005;166(5):733–739. doi:10.1086/431808.
- Sjb O, Poulis BAD, von Aderkas P. The identification of two thaumatin-like proteins (TLPs) in the pollination drop of hybrid yew that may play a role in pathogen defence during pollen collection. Tree Physiol. 2007;27(12):1649–1659. doi:10.1093/treephys/27.12.1649.
- Wagner RE, Mugnaini S, Sniezko R, Hardie D, Poulis B, Nepi M, Pacini E, von Aderkas P. Proteomic evaluation of gymnosperm pollination drop proteins indicates highly conserved and complex biological functions. Sex Plant Reprod. 2007;20(4):181–189. doi:10.1007/s00497-007-0054-8.
- Coulter A, Poulis BAD, von Aderkas P. Pollination drops as dynamic apoplastic secretions. Flora. 2012;207(7):482–490. doi:10.1016/j.flora.2012.06.004.
- Moffatt B, Ewart V, Eastman A. Cold comfort: plant antifreeze proteins. Physiol Plant. 2006;126(1):5–16. doi:10.1111/j.1399-3054.2006.00618.x.
- Passardi F, Cosio C, Penel C, Dunand C. Peroxidases have more functions than a swiss army knife. Plant Cell Rep. 2005;24(5):255–265. doi:10.1007/s00299-005-0972-6.
- Jin B, Jiang XX, Wang D, Zhang L, Wan YL, Wang L. The behavior of pollination drop secretion in Ginkgo biloba L. Plant Signal Behav. 2012a;7(9):1168–1176. doi:10.4161/psb.21122.
- Jin B, Zhang L, Lu Y, Wang D, Jiang XX, Zhang M, Wang L. The mechanism of pollination drop withdrawal in Ginkgo biloba L. BMC Plant Biol. 2012b;12(1):59. doi:10.1186/1471-2229-12-59.
- Jia B, Zhang SL. The comparison of the hormones and mineral elements contents among pollen, style and ovary in pear. Acta Hortic Sin. 2012;39(2):225–233. doi:10.16420/j.issn.0513-353x.2012.02.009.
- Malho R, Trewavas AJ. Localized apical increases of cytosolic free calcium control pollen tube orientation. Plant Cell. 1996;8(11):1935–1949. doi:10.2307/3870403.
- Wang Q, Lu L, Wu X, Li Y, Lin J. Boron influences pollen germination and pollen tube growth in Picea meyeri. Tree Physiol. 2003;23(5):345–351. doi:10.1093/treephys/23.5.345.
- Li J, Chen JZ, Huang YJ, Liang CH, Huang JC, Zhao CX, Zhou JH. Advances in research on physiology and metabolism of Zinc nutrition in fruit tree. J Fruit Sci. 2011;28(4):668–673. doi:10.13925/j.cnki.gsxb.2011.04.028.
- Wu JY, Jin C, Sl Z. Potassium flux in the pollen tubes was essential in plant sexual reproduction. Plant Signal Behav. 2011;6(6):898–900. doi:10.4161/psb.6.6.15322.
- Johnson DC, Dean DR, Smith AD, Johnson MK. Structure, function, and formation of biological iron-sulfur clusters. Annu Rev Biochem. 2005;74(74):247–281. doi:10.1146/annurev.biochem.74.082803.133518.
- Nicolson SW, Nepi M, Pacini E. Nectar consumers. In: Nicolson SW, Nepi M, Pacini E, editors. Nectaries and Nectar. Dordrecht(Germany): Springer; 2007. p. 289–342.
- Roy R, Schmitt AJ, Thomas JB, Carter CJ. Review: nectar biology: from molecules to ecosystems. Plant Sci. 2017;262:148–164. doi:10.1016/j.plantsci.2017.04.012.
- Jackson S, Nicolson SW. Xylose as a nectar sugar: from biochemistry to ecology. Biochem Mol Biol. 2002;131(4):613–620. doi:10.1016/S1096-4959(02)00028-3.
- Owens JN, Simpson SJ, Caron GE. The pollination mechanism of Engelmann spruce (Picea engelmannii). Can J Bot. 1987;65(7):1439–1450. doi:10.1139/b87-199.
- Prior N, Little SA, Pirone C, Gill JE, Smith D, Han J, Hardie D, Sjb O, Wagner RE, Cross T, et al. Application of proteomics to the study of pollination drops. Appl Plant Sci. 2013;1(4):1300008. doi:10.3732/apps.1300008.
- von Aderkas P, Prior NA, Gagnon S, Little S, Cross T, Hardie D, Borchers C, Thornburg R, Hou C, Lunny A. Degradome and secretome of pollination drops of Ephedra. Bot Rev. 2015;81(1):1–27. doi:10.1007/s12229-014-9147-x.
- Pirone-Davies C, Prior N, von Aderkas P, Smith D, Hardie D, Friedman WE, Mathews S. Insights from the pollination drop proteome and the ovule transcriptome of Cephalotaxus at the time of pollination drop production. Ann Bot. 2016;117(6):973–984. doi:10.1093/aob/mcw026.
- Edwards M, Bowman YJ, Dea IC, Reid JS. A beta-D-galactosidase from nasturtium (Tropaeolum majus L.) cotyledons: purification, properties, and demonstration that xyloglucan is the natural substrate. J Biol Chem. 1988;263(9):4333–4337.
- Sampedro J, Sieiro C, Revilla G. Cloning and expression pattern of a gene encoding an a-xylosidase active against xyloglucan oligosaccharides from Arabidopsis. Plant Physiol. 2001;126(2):910–920. doi:10.1104/pp.126.2.910.
- Dumbrepatil A, Park JM, Jung TY, Song HN, Jang MU, Han NS, Kim TJ, Woo EJ. Structural analysis of α-L-Arabinofuranosidase from Thermotoga maritima reveals characteristics for thermostability and substrate specificity. J Microbiol Biotechnol. 2012;22(12):1724–1730. doi:10.4014/jmb.1208.08043.
- Saha BC. α-l-Arabinofuranosidases. Biotechnol Adv. 2000;18(5):403–423. doi:10.1016/S0734-9750(00)00044-6.
- Rafińska K, Bednarska E. Localisation pattern of homogalacturonan and arabinogalactan proteins in developing ovules of the gymnosperm plant Larix decidua Mill. Sex Plant Reprod. 2011;24(1):75–87. doi:10.1007/s00497-010-0154-8.
- O’Leary SJB, Joseph C, von Aderkas P. Origin of arabinogalactan proteins in the pollination drop of Taxus x media. Aust J For Sci. 2004;121:35–46.
- Jr SH. Plant metabolism of xenobiotics. Trends Biochem Sci. 1992;17(2):82–84. doi:10.1016/0968-0004(92)90507-6.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(s 1–2):248–254. doi:10.1006/abio.1976.9999.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi:10.1038/227680a0.