ABSTRACT
Plants producing sufficient amount of aphid alarm pheromone by expressing (E)-β-Farnesene (EβF) synthase gene may contribute to plant protection by reducing aphid populations. However, terpene biosynthesis varies among plant species and developmental stages. In the present study, volatile headspace analysis of tobacco seedlings with MaβFS1 (an EβF synthase from the Asian peppermint Mentha asiatica) failed to generate EβF. We further targeted MaβFS1 to the tobacco plastid, using a chloroplast targeting sequence, either with or without the AtFPS1 gene for the biosynthesis of the precursor farnesyl diphosphate. When both MaβFS1 and AtFPS1 genes were targeted to the chloroplast, low levels of EβF were detected in stably transformed tobacco seedlings; resulting in specific repellence of the green peach aphid, Myzus persicae. These data indicate that redirecting the EβF biosynthetic pathway from its natural cytosolic location to the chloroplast is a valid strategy. This redirecting strategy may be very useful for other crop plants that do not naturally produce EβF or other repellent volatiles.
Aphids are globally distributed and among the most economically important agricultural pests, due to their direct ingestion of phloem sap, production of honeydew, and transmission of various phytopathogenic viruses.Citation1-Citation3 Insect pheromones, such as the aphid alarm pheromone, have been widely considered as potential alternatives to current pesticides, and are applicable for various integrated pest management (IPM) programs.Citation4-Citation6 (E)-β-farnesene (EβF) is the main or sole component of the alarm pheromone for most species of aphids. EβF may cause dispersal and repellence of aphids, as well as increase foraging by natural enemies including parasitic wasps and predators (e.g. ladybird beetles, syrphids, and lacewings).Citation4,Citation7 Interestingly, EβF also occurs in the volatiles of several plant species that are either constitutively emitting EβF and/or do so in response to herbivore. For caterpillar-damaged maize and trichomes of the wild potato Solanum berthaultii under natural conditions, the released EβF could act as an aphid deterrent.Citation8,Citation9
EβF synthase enzyme gene that catalyzes the conversion of the cytosolic farnesyl diphosphate (FPP) to EβF has been identified in several plant species, including Yuzu Citrus junos,Citation10 Douglas fir Pseudotsuga menziesii,Citation11 sweet wormwood Artemisia annua,Citation12,Citation13 chamomile Matricaria recutita,Citation14 and peppermint (Mentha piperita and Mentha asiatica).Citation15-Citation17 Following the identification of EβF synthase genes, efforts were made to reduce aphid infestation through genetic engineering. Expression of an EβF synthase from M. piperita, enabled Arabidopsis plants to produce EβF to keep the pest aphid Myzus persicae away and to increase the foraging behavior of Diaeretiella rapae.Citation18 Similar results have been documented for transgenic tobacco,Citation13,Citation17,Citation19 rice,Citation20 wheat,Citation4 Indian mustard Brassica juncea,Citation21 and alfalfa Medicago sativa.Citation22
Mechanisms of terpene biosynthesis vary among plant species and developmental stages.Citation23-Citation26 Previous studies documented that transgenic tobacco at the flowering stage produced small amounts of EβF.Citation13,Citation17,Citation19 Younger plants are more susceptible to aphids in nature, and during the young plant stage, aphid infestations significantly increase the economic losses of crop plants in the field.Citation2 In this context, we studied whether tobacco seedlings could generate sufficient EβF to repel aphids. Volatiles from both the control and MaβFS1 (an EβF synthase gene isolated from M. asiatica) seedlings were collected and analyzed via GC-MS. GC-MS analysis showed that the profile of MaβFS1 seedlings was similar to that of control seedlings, and no unique EβF peak was observed (Figure S1), indicating that MaβFS1 seedlings did not emit a detectable amount of EβF.
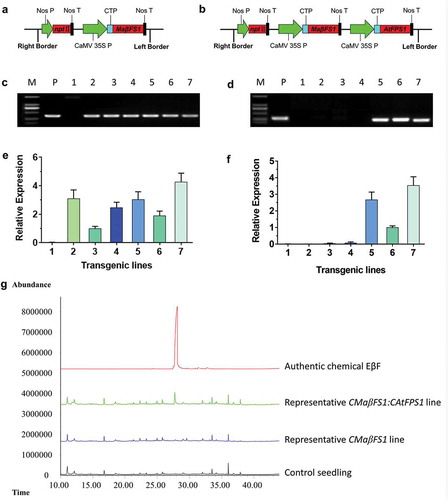
The precursor of sesquiterpenes FPP is naturally located in the cytosol; however, the plastid-isoform FPP synthase has been reported to occur in some species such as Arabidopsis, tomato, tobacco, rice, and wheat.Citation27-Citation29 Plant plastids (e.g. mitochondria and chloroplast) are an ideal compartment for satisfactory biosynthesis of sesquiterpenes via the retargeting of one or multiple biosynthetic enzymes, because of the high flux of IPP and DMAPP from MEP pathway in this organelle.Citation7,Citation30,Citation31 We proposed targeting sesquiterpene synthases into plastids to increase the EβF biosynthesis in our previous reviews.Citation2,Citation7 Here, two chloroplast-targeting constructs [CMaβFS1-pBI121 and CMaβFS1:CAtFPS1-pBI121 ())] were transferred into the tobacco. A total of 12 transgenic events with CMaβFS1-pBI121 and eight events with CMaβFS1:CAtFPS1-pBI121 were obtained. Positive lines from the T0–T2 generations were confirmed by PCR. Expression of the genes in T2 generations was confirmed via qRT-PCR with the primer pairs listed in table S1. Out of these, we chose three lead T2 lines each of CMaβFS1 (CMa2, CMa6, and CMa11) and CMaβFS1:CAtFPS1 lines (CMaCAt3, CMaCAt4, and CMaCAt7; ) to conduct GC-MS analyses. Tobacco seedlings expressing chloroplast-targeted forms of MaβFS1 plus AtFPS1 (CMaβFS1:CAtFPS1 lines) exhibited a unique EβF peak that was not present in the control ()). The emission levels of EβF from CMaCAt3, CMaCAt4, and CMaCAt7 lines were up to 4.43 ng/d per g fresh tissues. In contrast, we did not detect EβF in the headspace of tobacco seedlings expressing the chloroplast form of MaβFS1 alone that was unexpected. These results indicate that redirecting the EβF biosynthesis pathway from cytosolic to plastid by overexpressing MaβFS1 plus AtFPS1 in the chloroplast of tobacco seedlings increases the amount of emitted EβF.
Figure 1. Generation of tobacco lines with plastid-form of MaβFS1 alone or MaβFS1 plus AtFPS1. a) Schematic of CMaβFS1 expression cassette. b) Schematic of CMaβFS1:CAtFPS1 expression cassette. Nos P, Nos promoter; Nos T, Nos terminator; CaMV 35S P, CaMV 35S promoter. c) PCR identification of CMaβFS1 in T2 lines. d) PCR identification of CAtFPS1 in T2 lines. e) Gene expression analysis of CMaβFS1 in T2 lines. f) Gene expression analysis of CAtFPS1 in T2 lines. M, DL2000 DNA marker; P, CMaβFS1:CAtFPS1-pBI121 plasmid; 1, control with the blank vector pBI121; 2–4, CMaβFS1 lines CMa2, CMa6, and CMa11, respectively; 5–7, CMaβFS1:CAtFPS1 lines CMaCAt3, CMaCAt4, and CMaCAt7, respectively. g) Volatile analysis of tobacco seedlings expressing plastid-form of MaβFS1 alone or MaβFS1 plus AtFPS1.
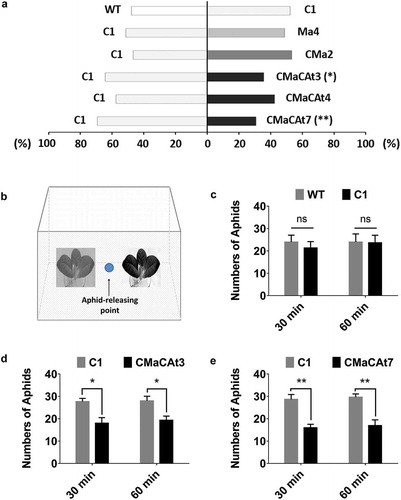
The continuous release of EβF in transgenic plant contributed to repelling aphids and reducing aphid colonization and plant damage.Citation13,Citation17-Citation22 In the present research, one MaβFS1 transgenic line Ma4, one CMaβFS1 transgenic line CMa2, and three CMaβFS1:CAtFPS1 transgenic lines (CMaCAt3, CMaCAt4, and CMaCAt7) were chosen for the Y-tube olfactometer assay. Y-tube results demonstrated that two CMaβFS1:CAtFPS1 transgenic seedlings (CMaCAt3 and CMaCAt7) were significantly repellent to alate aphids (); P < 0.05 or P < 0.01, χCitation2-test). Compared with untransformed and control tobacco seedlings, no different behavioral activity to entrainment volatiles of MaβFS1 and CMaβFS1 lines was detected (); P > 0.05, χCitation2-test). Further bioassays were performed with each of the transgenic and control seedlings placed in a 30 × 30 × 30 cm insect cage as indicated in ), with introduction of 50 alate aphids that were starved for 2 h. The number of aphids on each plant was counted after 30 and 60 min, respectively, with the aphids staying on the net cover were recorded as no choice. Compared with the control at the time-point of 30 min, aphids on CMaCAt3 and CMaCAt7 were decreased by about 34.9% (P < 0.05, t-test, n = 3) and 44.2% (P < 0.01, t-test, n = 3), respectively ()). At the 60 min, the number of aphids was reduced by approximately 31.0% in CMaCAt3 (P < 0.05, t-test, n = 3), and 42.7% in CMaCAt7 (P < 0.01, t-test, n = 3), respectively ()). The CMaβFS1:CAtFPS1 transgenic seedlings present here exhibited a repellent effect to minimize aphid infestation.
Figure 2. Preference of green peach aphid Myzus persicae on transgenic and control tobacco seedlings. WT, wild type tobacco seedling; C1, control seedling with the blank vector pBI121; Ma4, representative line of MaβFS1 seedling; CMa2, representative line of CMaβFS1 seedling; CMaCAt3, CMaCAt4, and CMaCAt7 are three representatives of CMaβFS1:CAtFPS1 lines. a) Behavioral response of M. persicae tested in a Y-tube olfactometer. The behavioral responses of 50 alate aphids to the volatiles collected from different transgenic lines were recorded. Choices between odor sources were analyzed with χ2-test. Asterisks indicate significant differences between treatments (*p < 0.05, **p < 0.01). ns indicates no significant difference at the 5% level (χ2-test). b) Schematic of the setup used for aphid repellence test in the greenhouse. Fifty alate aphids were placed in the midpoint circle at the start of each assay. The number of aphids was counted after 30 min and 60 min, respectively. c) The repellence test between wild type and control seedlings. Values shown in the figure are means ± SEM from three biological replicates. ns indicates no significant difference at the 5% level (t-test). d) The repellence test between control and CMaCAt3 seedlings. Values shown in the figure are means ± SEM. Single asterisk indicates significant differences at the 5% level between treatments (t-test). e) The repellence test between control and CMaCAt7 seedlings. Asterisks indicate significant differences between treatments (t-test; **P < 0.01).
The data reported in this paper strongly support our hypothesis that plastid-engineered tobacco seedlings generate sufficient EβF to repel aphids. Several points are germane. First, MaβFS1 seedlings did not emit a detectable amount of EβF. Second, targeting sesquiterpene synthases to plastids leads to demonstrable increases in EβF emissions. Third, aphid bioassays document aphid repellency in two engineered tobacco lines, CMaCAt3 and CMaCAt7. Taken together, these points amount to a potent argument that plastid-engineered crop plants may produce ecologically relevant emissions of pest-repellent volatiles. We recognize, however, that EβF and other plant volatile emissions highly volatile. Broadly considered, the volatile nature of the emissions is a necessary pre-condition for chemical communication between insects and their potential herbivores. It follows that the volatility leads to rapid dilution of the chemicals into the environment, and effective communication requires substantial biosynthesis and continued release of the volatiles. We note, too, that many plant volatiles are susceptible to oxidation,Citation32 which also drives selection for high biosynthesis and release. For deployment in agriculture, GM plants must release sufficient levels of EβF to cause the natural aphid alarm repellency response. In the context of field-level applications, our work has developed a model system for targeting enzymes for biosynthesis of plant volatiles to plastids. Future research should focus on improving the model to achieve high volatile production in crop plants for pest management. For a specific example, other multipoint metabolic engineering strategies to increase carbon flux through the mevalonate (MVA) or MEP pathway leading to FPP and EβF synthesis remain to be evaluated.
Supplemental Material
Download MS Word (178.5 KB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Yu XD, Liu ZC, Huang SL, Chen ZQ, Sun YW, Duan PF, Ma YZ, Xia LQ. RNAi-mediated plant protection against aphids. Pest Manag Sci. 2016;72:1090–1098. doi:10.1002/ps.4066.
- Yu XD, Wang GP, Huang SL, Ma YZ, Xia LQ. Engineering plants for aphid resistance: current status and future perspectives. Theor Appl Genet. 2014;127:2065–2083. doi:10.1007/s00122-014-2371-2.
- Jia DY, Gao SQ, Duan PF, Chen JB, Tian FX, Yu XD. Metabolic engineering of (E)-β-farnesene synthase genes for aphid-resistant genetically modified plants. Chin J Biotech. 2018;34:12–23.
- Bruce TJ, Aradottir GI, Smart LE, Martin JL, Caulfield JC, Doherty A, Sparks CA, Woodcock CM, Birkett MA, Napier JA. The first crop plant genetically engineered to release an insect pheromone for defence. Sci Rep. 2015;5:11183. doi:10.1038/srep11183.
- Xu Q, Hatt S, Lopes T, Zhang Y, Bodson B, Chen J, Francis F. A push–pull strategy to control aphids combines intercropping with semiochemical releases. J Pest Sci. 2018;91:93–103. doi:10.1007/s10340-017-0888-2.
- Zhou H, Chen L, Liu Y, Chen J, Francis F. Use of slow-release plant infochemicals to control aphids: a first investigation in a Belgian wheat field. Sci Rep. 2016;6:31552. doi:10.1038/srep31552.
- Yu XD, Pickett J, Ma YZ, Bruce T, Napier J, Jones HD, Xia LQ. Metabolic engineering of plant-derived (E)-β-farnesene synthase genes for a novel type of aphid-resistant genetically modified crop plants. J Integr Plant Biol. 2012;54:282–299. doi:10.1111/j.1744-7909.2012.01107.x.
- Bernasconi ML, Turlings TC, Ambrosetti L, Bassetti P, Dorn S. Herbivore-induced emissions of maize volatiles repel the corn leaf aphid, Rhopalosiphum maidis. Entomol Exp App. 1998;87:133–142. doi:10.1046/j.1570-7458.1998.00315.x.
- Gibson R, Pickett J. Wild potato repels aphids by release of aphid alarm pheromone. Nature. 1983;302:608–609. doi:10.1038/302608a0.
- Maruyama T, Ito M, Honda G. Molecular cloning, functional expression and characterization of (E)-β-farnesene synthase from Citrus junos. Biol Pharm Bull. 2001;24:1171–1175. doi:10.1248/bpb.24.1171.
- Huber DP, Philippe RN, Godard KA, Sturrock RN, Bohlmann J. Characterization of four terpene synthase cDNAs from methyl jasmonate-induced Douglas-fir, Pseudotsuga menziesii. Phytochemistry. 2005;66:1427–1439. doi:10.1016/j.phytochem.2005.04.030.
- Picaud S, Brodelius M, Brodelius PE. Expression, purification and characterization of recombinant (E)-β-farnesene synthase from Artemisia annua. Phytochemistry. 2005;66:961–967. doi:10.1016/j.phytochem.2005.03.027.
- Yu XD, Jones HD, Ma YZ, Wang GP, Xu ZS, Zhang BM, Zhang YJ, Ren GW, Pickett JA, Xia LQ. (E)-β-farnesene synthase genes affect aphid (Myzus persicae) infestation in tobacco (Nicotiana tabacum). Funct Integr Genomics. 2012;12:207–213. doi:10.1007/s10142-011-0244-1.
- Su S, Liu X, Pan G, Hou X, Zhang H, Yuan Y. In vitro characterization of a (E)-β-farnesene synthase from Matricaria recutita L. and its up-regulation by methyl jasmonate. Gene. 2015;571:58–64. doi:10.1016/j.gene.2015.06.037.
- Crock J, Wildung M, Croteau R. Isolation and bacterial expression of a sesquiterpene synthase cDNA clone from peppermint (Mentha x piperita, L.) that produces the aphid alarm pheromone (E)-β-farnesene. Proc Natl Acad Sci USA. 1997;94:12833–12838. doi:10.1073/pnas.94.24.12833.
- Prosser IM, Adams RJ, Beale MH, Hawkins ND, Phillips AL, Pickett JA, Field LM. Cloning and functional characterisation of a cis-muuroladiene synthase from black peppermint (Mentha x piperita) and direct evidence for a chemotype unable to synthesise farnesene. Phytochemistry. 2006;67:1564–1571. doi:10.1016/j.phytochem.2005.06.012.
- Yu XD, Zhang YJ, Ma YZ, Xu ZS, Wang GP, Xia LQ. Expression of an (E)-β-farnesene synthase gene from Asian peppermint in tobacco affected aphid infestation. The Crop J. 2013;1:50–60. doi:10.1016/j.cj.2013.07.005.
- Beale MH, Birkett MA, Bruce TJA, Chamberlain K, Field LM, Huttly AK, Martin JL, Parker R, Phillips AL, Pickett JA, et al. Aphid alarm pheromone produced by transgenic plants affects aphid and parasitoid behaviour. Proc Natl Acad Sci USA. 2006;103:10509–10513. doi:10.1073/pnas.0603998103.
- Wang GP, Yu XD, Fan J, Wang CS, Xia LQ. Expressing an (E)‐β‐farnesene synthase in the chloroplast of tobacco affects the preference of green peach aphid and its parasitoid. J Integr Plant Biol. 2015;57:770–782. doi:10.1111/jipb.12319.
- Gao L, Zhang X, Zhou F, Chen H, Lin Y. Expression of a peppermint (E)-β-Farnesene synthase gene in rice has significant repelling effect on bird cherry-oat aphid (Rhopalosiphum padi). Plant Mol Biol Rep. 2015;33:1967–1974. doi:10.1007/s11105-015-0888-4.
- Verma SS, Sinha RK, Jajoo A. (E)-β-farnesene gene reduces Lipaphis erysimi colonization in transgenic Brassica juncea lines. Plant Signal Behav. 2015;10:e1042636. doi:10.1080/15592324.2015.1030100.
- Wang X, Gao Y, Chen Z, Li J, Huang J, Cao J, Cui M, Ban L. (E)-β-farnesene synthase gene affects aphid behavior in transgenic Medicago sativa. Pest Manag Sci. 2018. doi:10.1002/ps.5153.
- Chen F, Tholl D, D’Auria JC, Farooq A, Pichersky E, Gershenzon J. Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers. Plant Cell. 2003;15:481–494. doi:10.1105/tpc.007989.
- Lavy M, Zuker A, Lewinsohn E, Larkov O, Ravid U, Vainstein A, Weiss D. Linalool and linalool oxide production in transgenic carnation flowers expressing the Clarkia breweri linalool synthase gene. Mol Breed. 2002;9:103–111. doi:10.1023/A:1026755414773.
- Mannan A, Ahmed I, Arshad W, Hussain I, Mirza B. Effects of vegetative and flowering stages on the biosynthesis of artemisinin in Artemisia species. Arch Pharm Res. 2011;34:1657–1661. doi:10.1007/s12272-011-1010-6.
- Muhlemann JK, Maeda H, Chang CY, San Miguel P, Baxter I, Cooper B, Perera MA, Nikolau BJ, Vitek O, Morgan JA. Developmental changes in the metabolic network of snapdragon flowers. PLoS One. 2012;7:e40381. doi:10.1371/journal.pone.0040381.
- Cunillera N, Boronat A, Ferrer A. The Arabidopsis thaliana FPS1 gene generates a novel mRNA that encodes a mitochondrial farnesyl-diphosphate synthase isoform. J Biol Chem. 1997;272:15381–15388. doi:10.1074/jbc.272.24.15381.
- Sallaud C, Rontein D, Onillon S, Jabès F, Duffé P, Giacalone C, Thoraval S, Escoffier C, Herbette G, Leonhardt N. A novel pathway for sesquiterpene biosynthesis from Z, Z-farnesyl pyrophosphate in the wild tomato Solanum habrochaites. Plant Cell. 2009;21:301–317. doi:10.1105/tpc.107.057885.
- Sanmiya K, Ueno O, Matsuoka M, Yamamoto N. Localization of farnesyl diphosphate synthase in chloroplasts. Plant Cell Physiol. 1999;40:348–354. doi:10.1093/oxfordjournals.pcp.a029549.
- Kappers IF, Aharoni A, van Herpen TWJM, Luckerhoff LLP, Dicke M, Bouwmeester HJ. Genetic engineering of terpenoid metabolism attracts bodyguards to Arabidopsis. Science. 2005;309:2070–2072. doi:10.1126/science.1116168.
- Wu S, Schalk M, Clark A, Miles RB, Coates R, Chappell J. Redirection of cytosolic or plastidic isoprenoid precursors elevates terpene production in plants. Nature Biotechnol. 2006;24:1441–1447. doi:10.1038/nbt1251.
- Hille Ris Lambers D, Schepers A. The effect of trans-\-farnesene, used as a repellant against landing aphid alatae in seed potato growing. Potato Res. 1978;21:23–26. doi:10.1007/BF02362255.
