ABSTRACT
The influences of stem flexure on shade-grown Serianthes nelsonii Merr. stem growth and strength were determined in a container nursery setting. Treated stems were bent 90° two times daily for a 14 wk nursery production period. Plant height, internode length, and slenderness were decreased by stem flexure when compared with control plants that received no flexure. Two force-displacement tests revealed stem strength was increased by the flexure treatment. Control plants exhibited undesirable lean of the main stem, and 1 hr of wind stress further increased the angle of lean. Treated plants were close to orthotropic and the wind stress did not change the stem lean. Results indicate stem flexure is a reliable method for increasing the quality of shade-grown S. nelsonii plants and some form of mechanical stimulation should be added to nursery production protocols for the species.
Introduction
Defining the most beneficial nursery protocols for endangered tree species can be challenging due to a dearth of direct experimentation on the model species.Citation1 However, the routine production of nursery plants for species recovery purposes need not ignore the potential for non-destructive nursery research that carries the potential to add new knowledge. Until recently,Citation2–Citation7 the Endangered Serianthes nelsoniiCitation8,Citation9 has received almost no serious research from conservationists despite the critical call for more research 25 years ago.Citation10 Therefore, the knowledge needed to define the best nursery management protocols remains obscure.
Container-grown plants of this late successional tree species exhibit greater stem growth when grown in shade.Citation2 However, taller and thinner structures are more at risk of mechanical failure,Citation11 and the main stem of the shade-grown plants tends to lodge in the nursery. This can lead to even more lodging after removal from the protected environment of the nursery. This undesirable trait of the shade-grown plants also leads to undesirable bud-break of lateral stems, and the plant subsequently develops an undesirable shrub-like appearance rather than retaining a small tree form (). One treatment that can improve overall growth and strength of the plants is repeated pruning, a treatment that also improves plant quality of the congeneric Serianthes grandiflora Benth and Serianthes kanehirae Fosberg.Citation6 But stronger horticultural nursery plants can also be produced by adding mechanical stimulation to nursery production protocols.Citation12
Figure 1. Shade-grown Serianthes nelsonii plants fail to maintain orthotropic stem orientation, and leaning leads to undesirable lateral bud break that reduces plant quality (yellow arrows).

My objective was to apply stem flexure to container-grown S. nelsonii seedlings to determine if this species is equipped to improve plant quality and stem strength in response to the treatment. All of the treatments and measurements were non-destructive to ensure the nursery plants were not harmed and could be added to a species recovery population.
Materials and methods
Seeds of S. nelsonii from the University of Guam conservation nursery were sown on 30 Sep 2014, and the seedlings were transplanted individually to 2.6 L containers on 24 Nov 2014 (mean height 147 cm, basal diameter 1.7 mm). Container medium, fertilization regime, and irrigation management were as previously described.Citation2,Citation6
The plants were placed randomly on a raised bench and grown under shadecloth with 45% sunlight transmission. Half of the plants were randomly designated as controls, and these plants received no mechanical stimulation. The other half of the plants were considered the treated plants, and stem bending was begun on 27 Nov 2014. For the bending treatment, the base of the stem was held with one hand and the stem was bent to a 90° angle 10 times with the other hand. The direction of bending was random to ensure a unidirectional thigmomorphogenesis response would not occur. The treatment was applied one time in the early morning, and one time in the late afternoon every day. The study was terminated when the plants within one of the treatments reached 50 cm in height, which occurred on 6 Mar 2015. Therefore, the plants were ~8 wk old when the treatments were begun, and the treatments were imposed for ~14 wk.
Plant height and basal stem diameter were measured. The influence of flexure on these traits was calculated by subtracting initial height and diameter from ending height and diameter. Slenderness was calculated as ending stem height/diameter. The length of the terminal five internodes was measured for each replication. The angle of the main stem was determined by holding a protractor on a bubble level and recording the angle. An angle of 0° in this study was designated as orthotropic, and an angle of 90° was designated as plagiotropic. Then, each plant was subjected to 1 hr of 2.9 m·s−1 unidirectional wind applied with electric fans. After the wind treatment, the angle of the main stem was re-measured using the same procedures.
Stem biomechanics were studied with two common force-displacement tests. The first test was carried out by placing each plant laterally with the container held in place such that a hydraulic jack supported by a balance was able to impose a displacement force with the loading anvil at 20 cm stem height. Lateral displacement was applied on 1 cm increments up to 10 cm displacement, and the force required for each displacement increment was recorded from the balance. The force in this test was transferred to the lower stem and roots of the plants. The second test was a three-point test, with two supporting anvils placed at 2 and 24 cm stem height, and the hydraulic jack positioned to provide a loading anvil at the midpoint. Lateral displacement was applied on 0.5 cm increments from 0.5 to 2.0 cm, and the force required for each displacement increment was recorded. The force in this test was transferred to the stems between the two supporting anvils only. Important biomechanical traits such as modulus of rupture were not pursued because the amplitude of displacement was kept to a minimum to ensure no damage to the stem occurred. Instead, the dependent variable force was regressed onto the independent variable displacement distance using the Proc GLM function in SAS (SAS Institute, Cary, Ind, USA) to obtain a linear coefficient for each replication.
The response variables were subjected to t test to determine the influence of stem flexure on height increment, diameter increment, slenderness, stem angle before and after wind, and the slope of the force–displacement curves for both biomechanical tests.
Results
Stem bending of S. nelsonii plants reduced stem height growth by 17% and internode length by 30% below that of control plants (). In contrast, a non-significant increase in stem diameter occurred following stem bending. These height and diameter responses generated a 23% decrease in slenderness following stem bending.
Table 1. The influence of twice-daily stem bending on morphometric traits of Serianthes nelsonii plants. Mean ± SE, n = 8.
The lean of the main stem of control plants at the end of the nursery production cycle was more than three times greater than that of the treated plants (). Following 1 hr of wind, the lean of the control plants more than doubled. In contrast, 1 hr of wind did not affect the lean of the treated plants.
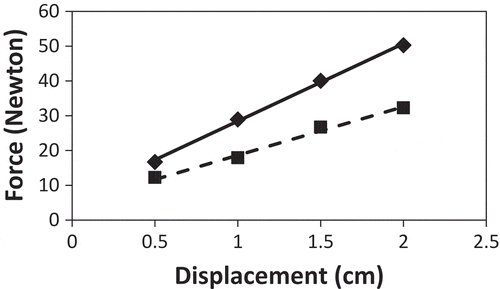
The force required to displace control plants at 20 cm height (slope of the displacement curve) was 37% lower than for the treated plants (). For control plants, the force to displace 1 cm and 2 cm was so low that it was below the precision of the balance. The force required to displace control plant stems in a three-point test was also 37% lower than that for the treated plants ().
Figure 2. The force required to displace Serianthes nelsonii stems as influenced by daily bending stress. Displacement height was 20 cm above the root collar. Treated plants (diamonds and solid line): y = −1.11 + 2.61x; control plants (squares and dashed line): y = −0.48 + 1.65x. Slopes of individual replications subjected to t test: t = 34.18, P < 0.0001.
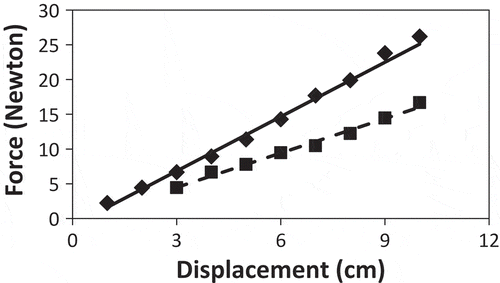
Figure 3. The force required to displace Serianthes nelsonii stems placed in a three-point test as influenced by daily bending stress. Fulcrum points were 22 cm apart, with point of force at the midpoint. Treated plants (diamonds and solid line): y = 6.17 + 22.29x; control plants (squares and dashed line): y = 4.68 + 13.95x. Slopes of individual replications subjected to t test: t = 17.17, P < 0.0001.
Discussion
The design of a plant is complicated by competing tradeoffs in costs and constraints.Citation13–Citation15 Indeed, life-history theory indicates that plants cannot maximize all fitness components, so adding an adaptive response to a plant’s survival toolkit usually comes at a cost. Increased biomechanical strength following mechanical stimulation of plants may be context-dependent and some plants may not possess the ability to improve morphology following mechanical stimuli. For example, a late successional species such as S. nelsonii produces seedlings and saplings that thrive in the forest understory where minimal wind stimulation occurs but competition for limited light and soil resources is severe. The need to possess the machinery to compete for these ubiquitous abiotic limitations in this setting may therefore cause small plants of this species to be unable to beneficially respond to uncharacteristic mechanical stimuli such as wind. These competing tradeoffs mean that there is no way to predict the manner in which a species will optimize its adaptive responses to mechanical stimulation without direct study.
I have shown that young S. nelsonii plants do indeed exhibit a rapid thigmomorphogenesis response that improves biomechanical properties and quality of the nursery plant. The benefits of changes in slenderness and stem strength were evident following the wind stress treatment, as the lean of the mechanically stimulated plants was not influenced by the wind stress. In contrast, the control plants succumbed to this wind stress with a resulting lean that was about 30° from vertical. This new knowledge may be used to develop some form of mechanical stimulation in a shaded nursery to improve the quality of the S. nelsonii transplants, which will in turn improve plant performance after removal from the conservation nursery for out-planting.
The endogenous signals that provoke thigmomorphogenesis in plants are not adequately understood. Various hormonesCitation16-Citation18 and calciumCitation19-Citation23 may be pivotal for generating the morphological and growth responses of plants to mechanical stimulation. A greater understanding of these factors may improve nursery protocols designed to exploit the benefits of thigmomorphogenesis.
Thigmomorphogenesis responses are duration- and dose-responsive.Citation24,Citation25 In this study, the twice-daily bending treatment generated a beneficial biomechanical response of S. nelsonii plants in a shaded nursery, improving slenderness and stem strength but reducing lodging. A similar response may be achievable with a lower dose level of the mechanical stimulation. Alternatively, a greater response may be achievable with a greater dose level or frequency. More research is required to determine the most efficient means of generating beneficial thigmomorphogenesis responses of this tree species. More research would likely reveal that a combination of repeated pruningCitation6 and mechanical stimulation would produce the highest quality S. nelsonii transplant.
The United States Endangered Species Act (ESA)Citation26 prohibits the damage of plants that are protected by the Act. Plants are damaged by experiencing stressors and stimuli that cause a decline in growth or quality, but they are also damaged by an inability to acquire resources or stimuli that are beneficial to growth or quality.Citation27,Citation28 The benefits of mechanical stimulation for producing high-quality nursery plants has been known for 46 yr,Citation12 and my findings indicate that S. nelsonii is among the species that benefit from this common horticultural treatment. Nursery practitioners of ESA-listed plant species should embrace the adaptive management approachCitation29 for improving horticultural treatment by learning from ongoing management outcomes. In this light, my findings indicate that practitioners need to add mechanical stimulation to the nursery production protocols for shade-grown S. nelsonii nursery plants. In contrast, practitioners who withhold the application of a mechanical stimulus (or a similar treatment that is later proven to strengthen the weak stems of shade-grown S. nelsonii transplants) are acting out of compliance of the ESA, as they are withholding a stimulus that is now known to produce high-quality nursery plants.
Acknowledgments
Nursery support provided by Cameron Musser and Nirmala Dongol. No take or collection was directly associated with this research.
References
- Marler TE. Horticultural research crucial for plant conservation and ecosystem restoration. HortScience. 2017;52:1648–1649. doi:10.21273/HORTSCI12423-17.
- Marler TE, Cascasan AN, Lawrence JH. Threatened native trees in Guam: short-term seed storage and shade conditions influence emergence and growth of seedlings. HortScience. 2015;50:1049–1054. doi:10.21273/HORTSCI.50.7.1049.
- Marler TE, Cascasan AN. Number of emerged seedlings and seedling longevity of the non-recruiting, critically endangered Håyun lågu tree Serianthes nelsonii Merr. (Fabales: leguminosae) are influenced by month of emergence. J Threatened Taxa. 2015;7:8221–8225. doi:10.11609/jott.2100.7.15.8221-8225.
- Marler T, Musser C. Potential stressors leading to seedling mortality in the endemic Håyun lågu tree (Serianthes nelsonii Merr.) in the island of Guam. Trop Cons Sci. 2015;8:738–744. doi:10.1177/194008291500800311.
- Marler T, Musser C. Chemical and air pruning of roots influence post-transplant root traits of the critically endangered Serianthes nelsonii. Plant Root. 2016;10:21–25. doi:10.3117/plantroot.10.21.
- Marler TE. Asexual reproduction to propel recovery efforts of the critically endangered Håyun Lågu tree (Serianthes nelsonii Merr.). Trop Cons Sci. 2017;10:1–10. doi:10.1177/1940082917697707.
- Marler TE. Diel root extension patterns of three Serianthes species are modulated by plant size. Plant Signal Behav. 2017;12:e1327496. doi:10.1080/15592324.2017.1327496.
- United States Fish & Wildlife Service. Endangered and threatened wildlife and plants; determination of endangered status for Serianthes nelsonii Merr. (Hayun Lagu or Tronkon Guafi). Fed Regist. 1987;52(32):4907–4910. Including correction of tabular error: Federal Register 52 (42):6651.
- Wiles G, Williams E. Serianthes nelsonii. The IUCN Red List of Threatened Species 2017: e.T30437A98715973. 2017; [accessed 2019 Mar 13]. doi:10.2305/IUCN.UK.2017-3.RLTS.T30437A98715973.en.
- United States Fish & Wildlife Service. Recovery plan for Serianthes nelsonii. Portland (Oregon): Fish & Wildlife Service; 1994.
- Niklas KJ. Plant biomechanics: an engineering approach to plant form and function. Chicago (Ill): University of Chicago Press; 1992.
- Jaffe MJ. Thigmomorphogenesis: the response of plant growth and development to mechanical stimulation. Planta. 1973;114:143–157. doi:10.1007/BF00387472.
- Read J, Stokes A. Plant biomechanics in an ecological context. Amer J Bot. 2006;93:1546–1565. doi:10.3732/ajb.93.10.1546.
- Anten NPR, von Wettberg EJ, Pawlowski M, Huber H. Interactive effects of spectral shading and mechanical stress on the expression and costs of shade avoidance. Amer Naturalist. 2009;173:241–255. doi:10.1086/595761.
- Badel E, Ewers FW, Cochard H, Telewski FW. Acclimation of mechanical and hydraulic functions in trees: impact of thigmomorphogenetic process. Front Plant Sci. 2015;6:266. doi:10.3389/fpls.2015.00266.
- Erner Y, Jaffe MJ. Thigmomorphogenesis: the involvement of auxin and abscisic acid in growth retardation due to mechanical perturbation. Plant Cell Physiol. 1982;23:935–941.
- Biro RL, Jaffe MJ. Thigmomorphogenesis: ethylene evolution and its role in the changes observed in mechanically perturbated bean plants. Physiol Plant. 1984;62:289–294. doi:10.1111/j.1399-3054.1984.tb04575.x.
- Telewski FW, Jaffe MJ. Thigmomorphogenesis: the role of ethylene in the response of Pinus taeda and Abis fraseri to mechanical perturbation. Physiol Plant. 1986;66:227–233. doi:10.1111/j.1399-3054.1986.tb02413.x.
- Braam J, Davis RW. Rain-, wind-, and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell. 1990;60:357–364. doi:10.1016/0092-8674(90)90587-5.
- Knight MR, Smith SM, Trewavas AJ. Wind-induced plant motion immediately increases cytosolic calcium. Proc Natl Acad Sci. 1992;89:4967–4971. doi:10.1073/pnas.89.11.4967.
- Trewavas A, Knight M. Mechanical signaling, calcium and plant form. Plant Mol Biol. 1994;26:1329–1341. doi:10.1007/BF00016478.
- Kurusu T, Kuchitsu K, Nakano M, Nakayama Y, Iida H. Plant mechanosensing and Ca2+ transport. Trends Plant Sci. 2013;18:227–233. doi:10.1016/j.tplants.2012.12.002.
- Huynh T-P, Haick H. Learning from intelligent mechanosensing system of plants. Adv Mater Technol. 2019;4:1800464. doi:10.1002/admt.201800464.
- Jaffe MJ, Biro R, Bridle K. Thigmomorphogenesis: calibration of the parameters of the sensory function in beans. Physiol Plant. 1980;49:410–416. doi:10.1111/ppl.1980.49.issue-4.
- Telewski RW, Pruyn ML. Thigmomorphogenesis: a dose response to flexing in Ulmus americana seedlings. Tree Physiol. 1998;18:65–68. doi:10.1093/treephys/18.1.65.
- United States Fish & Wildlife Service. Endangered Species Act of 1973. Washington (D.C.): Department of the Interior. [accessed 2019 Mar 13]. http://www.fws.gov.
- Tuteja N, Gill SS, eds. Abiotic stress response in plants. Weinheim (Germany): Wiley-VCH; 2016.
- Shabala S, ed. Plant stress physiology. Oxfordshire (UK): CABI Press; 2017.
- Holling CS. Adaptive environmental assessment and management. New York (NY): John Wiley & Sons; 1978.
