ABSTRACT
γ-Aminobutyric acid (GABA) is an important neurotransmitter in mammals whose receptor is reported to be regulated by flavonoids. In plants, it is considered to be at the intersection of carbon and nitrogen metabolism, but its relationship with flavonoid metabolism remains unclear. Our recent RNA-seq analysis showed that expression of flavonoid biosynthetic genes was influenced in poplar by the blockage of α-ketoglutarate dehydrogenase (α-KGDH) activity and the application of GABA under NaCl stress, accompanied by the changes in GABA shunt activity. Here, we further found that the flavonoid accumulation was significantly affected by blocking the activities of α-KGDH and GABA transaminase as well as applying exogenous GABA, coupled with the changes of endogenous GABA contents. Key genes involved in the flavonoid biosynthetic pathway were also significantly influenced, including two PALs, 4CL, and two CHSs. Our results suggest that the GABA shunt is closely associated with the metabolism of flavonoids, which would benefit future understanding of GABA’s roles in carbon allocation by regulating the pathway of flavonoid biosynthesis under normal or stress conditions.
γ-Aminobutyric acid (GABA) is a unique four-carbon non-protein amino acid that was first identified in potato tubers in 1949Citation1; subsequently, it was discovered in mammalian brainsCitation2 where it functions as a neurotransmitter with multiple receptors.Citation3 In plants, GABA is rapidly accumulated in response to abiotic and biotic stresses.Citation4–Citation8 GABA is mainly synthesized and metabolized via the GABA shunt, which bypasses two enzymatic reactions in the tricarboxylic acid (TCA) cycle. Therefore, the GABA shunt is located at a key position between carbon and nitrogen metabolism,Citation6–Citation8 and the latter is closely associated with secondary metabolism.Citation9
Flavonoids are a type of polyphenol, a plant secondary metabolite,Citation10,Citation11 that is derived from the phenylpropanoid pathway.Citation12 They are closely associated with carbon metabolismCitation13 and nitrogen metabolism.Citation14 Previous studies demonstrated that exogenous GABA significantly increases the accumulation of phenolic compounds in barleyCitation15 and that of flavonoids in turnip.Citation16 Furthermore, our recent RNA-seq analysis showed that changes in endogenous GABA levels can affect the expression of genes involved in the flavonoid biosynthetic pathway in the leaves or roots of poplar, revealed by inhibiting α-ketoglutarate dehydrogenase (α-KGDH) activity in the TCA cycle using the specific and irreversible inhibitor succinyl phosphonate (SP)Citation17 as well as by applying GABA under NaCl stress,Citation5 suggesting a potential relationship between GABA and flavonoids.
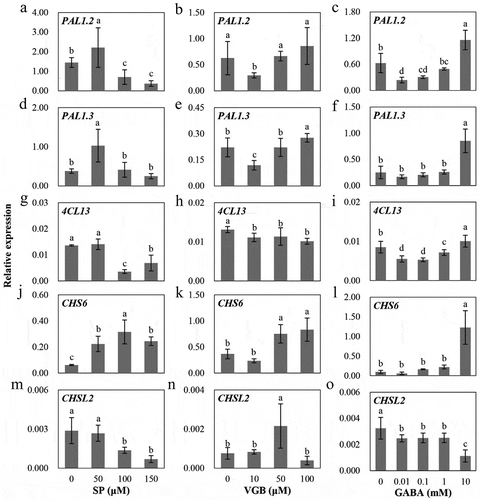
Therefore, in the present study, we further investigated the effects of manipulating the GABA shunt on flavonoid metabolism in the leaves following treatment with exogenous SP (0, 50, 100, and 150 μM),Citation17 vigabatrin (VGB; a specific inhibitor of GABA transaminase/GABA-T; 0, 10, 50, and 100 μM), and GABA (0, 0.01, 0.1, 1, and 10 mM) for 12 d after the stem segments, from one-month tissue-cultured poplar seedlings, were separately sub-cultured on 1/2 Murashige and Skoog medium. The inhibition of α-KGDH activity reduced the total flavonoid concentrations in leaves ()), coupled with a decrease in endogenous GABA levels as shown in our previous work.Citation17 However, the blockage of GABA-T activity and exogenous GABA evidently enhanced the total flavonoid concentrations (), accompanied by the increase of endogenous GABA levels in leaves (data not shown). This result was consistent with that previously seen following exogenous GABA treatment of turnip.Citation16 The expression of genes involved in the flavonoid biosynthetic pathway was significantly affected after inhibiting α-KGDH and GABA-T as well as exogenous GABA (). Two PAL genes (PAL1.2 and PAL1.3), encoding phenylalanine ammonia-lyase, were significantly upregulated after treatment with 50 μM SP and significantly downregulated or rescued to control levels with 100 and 150 μM SP treatments. They showed the opposite trends under VGB and GABA treatments, being significantly downregulated at lower concentrations but upregulated at higher concentrations although there were no significant differences in certain concentrations (). 4CL13, encoding 4-coumarate-CoA ligase which is located at the critical cleavage site of the phenylpropanoid pathway,Citation19 was decreased after almost all treatments of SP, VGB, and GABA except for a significant increase at 10 mM GABA (). This is similar to previous findings of expression changes to PAL and 4CL in barley under NaCl stress after the external application of GABA.Citation15 CHS6 and CHSL2, encoding chalcone synthases which are the first committed enzymes in flavonoid biosynthesis,Citation11 almost showed the opposite expression trends: CHS6 was found to be upregulated, while CHSL2 was downregulated by all three treatments, except that CHSL2 was still upregulated by VGB treatment (). A close observation found that CHSL2 had much lower expression levels although it was significantly affected by all the treatments (). These results indicate that changes in endogenous GABA concentrations almost had a positive relationship with flavonoid accumulation although they showed a bit of discrepancies at transcriptional levels.
Figure 1. Effects of exogenous SP (a), VGB (b), and GABA (c) on flavonoid accumulation in leaves of poplar seedlings at 12 d. Leafy stem segments from one-month-old 84K poplar (Populus alba × P. glandulosa cv. 84K) were propagated on 1/2 Murashige and Skoog (MS) medium solidified with 1.0% agar containing SP (0, 50, 100, or 150 μM; MedChem Express, Monmouth, NJ, USA) or VGB (0, 10, 50, or 100 μM; MCE, Monmouth Junction, NJ, USA) or GABA (0, 0.01, 0.1, 1, or 10 mM; Sigma–Aldrich, St. Louis, MO, USA) and cultured in a greenhouse set at 25°C under a 16-h light/8-h dark cycle for 12 d. The leaves of three biological repeats were sampled to measure the flavonoid contents according to our previous work.Citation18 Post hoc analysis was performed by Duncan‘s multiple range test in one-way ANOVA method of SPSS 16.0 software, and lowercase letters indicate statistically significant differences among the treatments (P< 0.05).
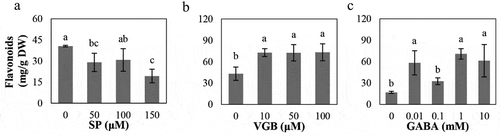
Figure 2. Expression changes of genes involved in flavonoid biosynthetic pathway in leaves at 12 d under different concentrations of SP or VGB or GABA. The methods of treatments are shown as in . Total RNA was isolated with the RNAprep Pure Plant Kit (Tiangen, Beijing, China) and then reverse transcribed to cDNA with the PrimeScript™ RT Reagent Kit (Takara, Otsu, Japan). The targeted genes were selected based on our published work.Citation17 PtoUBQ was used as the internal reference gene. The leaves of three biological repeats were sampled to analyze the gene expression by qRT-PCR. The method of the statistical analysis is shown as in .
Flavonoids have been shown to coordinate the plant response to a variety of biotic and abiotic stresses,Citation20 which also affect carbon allocation.Citation14,Citation21 Combined with our previous results,Citation5,Citation17 the current finding showed that GABA can regulate flavonoid production, indicating that the GABA shunt might participate in regulating carbon allocation by affecting the flavonoid biosynthesis pathway under normal or stress conditions. On the other hand, flavonoids interact with GABA receptors in the central nervous system of mammals.Citation22,Citation23 Hence, it would be worthwhile investigating whether they have similar functions in relation to the recently identified plant GABA receptor.Citation24 Our future work will explore the relationship between GABA and flavonoid metabolism by overexpressing or knockout GADs in poplar.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Fundamental Research Funds for the Central Non-profit Research Institution of CAF (CAFYBB2016SY002; CAFYBB2014ZX001-3), the National Natural Science Foundation of China (31100490), Fund Project of Science and Technology Activities for Overseas Students of the Ministry of Human Resources and Social Sciences Security of the People’s Republic of China (2016). We also thank Sarah Williams, PhD, from Liwen Bianji, Edanz Group China, for editing the English text of a draft of this manuscript.
Additional information
Funding
References
- Steward FC, Thompson JF, Dent CE. γ-Aminobutyric acid: a constituent of potato tubers? Science. 1949;110:439–440.
- Roberts E, Frankel S. γ-Aminobutyric acid in brain: its formation from glutamic acid. J Biol Chem. 1950;187:55–63. PMID:14794689.
- Johnston GAR. GABA Australis, some reflections on the history of GABA receptor research in Australia. Pharmacol Res. 2017;116:32–38. PMID:28017666. doi:10.1016/j.phrs.2016.12.031.
- Michaeli S, Fait A, Lagor K, Nunes-Nesi A, Grillich N, Yellin A, Bar D, Khan M, Fernie AR, Turano FJ, et al. A mitochondrial GABA permease connects the GABA shunt and the TCA cycle, and is essential for normal carbon metabolism. Plant J. 2011;67:485–498. PMID: 21501262. doi:10.1111/j.1365-313X.2011.04612.x.
- Ji J, Yue J, Xie T, Chen W, Du C, Chang E, Chen L, Jiang Z, Shi S. Roles of gamma-aminobutyric acid on salinity-responsive genes at transcriptomic level in poplar: involving in abscisic acid and ethylene-signalling pathways. Planta. 2018;248:675–690. PMID:29948123. doi:10.1007/s00425-018-2915-9.
- Fait A, Fromm H, Walter D, Galili G, Fernie AR. Highway or byway: the metabolic role of the GABA shunt in plants. Trends Plant Sci. 2008;13:14–19. PMID:18155636. doi:10.1016/j.tplants.2007.10.005.
- Bown AW, Shelp BJ. Plant GABA: not just a metabolite. Trends Plant Sci. 2016;21:811–813. PMID:27542324. doi:10.1016/j.tplants.2016.08.001.
- Michaeli S, Fromm H. Closing the loop on the GABA shunt in plants: are GABA metabolism and signaling entwined? Front Plant Sci. 2015;6:419. PMID:26106401. doi:10.3389/fpls.2015.00419.
- Jensen RA. The shikimate/arogenate pathway: link between carbohydrate metabolism and secondary metabolism. Physiol Plant. 1985;66:164–168. doi:10.1111/j.1399-3054.1986.tb01251.x.
- Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2:1231–1246. PMID:22254006. doi:10.3390/nu2121231.
- Vogt T. Phenypropanoid biosynthesis. Mol Plant. 2010;3:2–20. PMID: 20035037. doi:10.1093/mp/ssp106.
- Lepiniec L, Debeaujon I, Routaboul JM, Baudry A, Pourcel L, Nesi N, Caboche M. Genetics and biochemistry of seed flavonoids. Annu Rev Plant Biol. 2006;57:405–430. PMID:16669768. doi:10.1146/annurev.arplant.57.032905.105252.
- Vidović M, Morina F, Milić S, Albert A, Zechmann B, Tosti T, Winkler JB, Jovanović SV. Carbon allocation from source to sink leaf tissue in relation to flavonoid biosynthesis in variegated Pelargonium zonale under UV-B radiation and high PAR intensity. Plant Physiol Biochem. 2015;93:44–55. PMID:25661975. doi:10.1016/j.plaphy.2015.01.008.
- Becker C, Urlić B, Špika MJ, Kläring HP, Krumbein A, Baldermann S, Goreta Ban S, Perica S, Schwarz D, Araujo WL. Nitrogen limited red and green leaf lettuce accumulate flavonoid glycosides, caffeic acid derivatives, and sucrose while losing chlorophylls, beta-carotene and xanthophylls. PLoS One. 2015;10: PMID:26569488. doi:10.1371/journal.pone.0142867.
- Ma Y, Wang P, Wang M, Sun M, Gu Z, Yang R. GABA mediates phenolic compounds accumulation and the antioxidant system enhancement in germinated hulless barley under NaCl stress. Food Chem. 2019;270:593–601. PMID:30174091. doi:10.1016/j.foodchem.2018.07.092.
- Thiruvengadam M, Kim SH, Chung IM. Influence of amphetamine, γ-aminobutyric acid, and fosmidomycin on metabolic, transcriptional variations and determination of their biological activities in turnip (Brassica rapa ssp. rapa). S Afr J Bot. 2016;103:181–192. doi:10.1016/j.sajb.2015.08.021.
- Yue J, Du C, Ji J, Xie T, Chen W, Chang E, Chen L, Jiang Z, Shi S. Inhibition of α-ketoglutarate dehydrogenase activity affects adventitious root growth in poplar via changes in GABA shunt. Planta. 2018;248:963–979. PMID:29982922. doi:10.1007/s00425-018-2929-3.
- Deng N, Chang E, Li M, Ji J, Yao X, Bartish IV, Liu J, Ma J, Chen L, Jiang Z, et al. Transcriptome characterization of Gnetum parvifolium reveals candidate genes involved in important secondary metabolic pathways of flavonoids and stilbenoids. Front Plant Sci. 2016;7:174. PMID:26973657. doi:10.3389/fpls.2016.00174.
- Duthie G, Crozier A. Plant-derived phenolic antioxidants. Curr Opin Clin Nutr Metab Care. 2000;11:447–451. PMID:11085830. doi:10.1097/00041433-200002000-00007.
- Mierziak J, Kostyn K, Kulma A. Flavonoids as important molecules of plant interactions with the environment. Molecules. 2014;19:16240–16265. PMID:25310150. doi:10.3390/molecules191016240.
- Gao Y, Li XX, Han MM, Yang XF, Li Z, Wang J, Pan QH. Rain-shelter cultivation modifies carbon allocation in the polyphenolic and volatile metabolism of Vitis vinifera L. Chardonnay Grapes. PLoS One. 2016;11. PMID:27218245. doi:10.1371/journal.pone.0156117.
- Hanrahan JR, Chebib M, Johnston GAR. Flavonoid modulation of GABAA receptors. Br J Pharmacol. 2011;163:234–245. PMID:21244373. doi:10.1111/j.1476-5381.2011.01228.x.
- Wasowski C, Marder M. Flavonoids as GABAA receptor ligands: the whole story? J Exp Pharmacol. 2012;4:9–24. PMID:27186113. doi:10.2147/JEP.S23105.
- Ramesh SA, Tyerman SD, Xu B, Bose J, Kaur S, Conn V, Domingos P, Ullah S, Wege S, Shabala S, et al. GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat Commun. 2015;6:7879. PMID:26219411. doi:10.1038/ncomms8879.
