ABSTRACT
The MAPK signaling cascade is universal among eukaryotes and mediates a variety of environmental and developmental responses. Two Arabidopsis MAPKs, MPK3 and MPK6, have been shown to be activated by various stimuli and suggested as a convergence point of different signaling pathways. It is known that these MAPKs, MPK3/MPK6, control the discrete stages of stomatal development in Arabidopsis, but how they are regulated and how the same MAPK components can achieve signaling specificity is largely unknown. We recently demonstrated that MAP Kinase Phosphatase 1 (MKP1) promotes stomatal differentiation by suppressing activation of MPK3/MPK6 in the stomatal lineage. By expressing MKP1 in discrete stomatal precursor cell types, we further identified that MKP1 plays an important role at the early stage of stomatal development for the cell fate transition leading to stomatal differentiation. While MKP1 was previously known as a key regulator of environmental stress responses, our data illustrate a novel role of MKP1 in plant development: it acts as one of the specificity-determining regulators of MAPK signaling to enforce proper stomatal development in Arabidopsis.
KEYWORDS:
Mitogen-activated protein kinase (MAPK) cascades form one of the key components of signal transduction in eukaryotes, a process in which they function to integrate and transduce extracellular stimuli into intracellular responses. As terminal components of a hierarchical series of sequential phosphorylation events, the activity of MAPKs is up-regulated through MAPKK-catalyzed phosphorylation and down-regulated through dephosphorylation catalyzed by phosphatases. In plants, activation of MAPK cascades has been associated with a wide range of stress, hormonal, and developmental responses including stomatal patterning and differentiation,Citation1,Citation2 but much less is known about the corresponding MAPK deactivation processes.
Stomata, which valves on the plant epidermis used for gas exchange with the atmosphere, are formed through a series of division and differentiation events. Proper patterning and differentiation of stomata is critical for their function and is enforced by MAPK-dependent processes.Citation2,Citation3 Interestingly, the same MAPKs, MPK3 and MPK6, have been shown to act in distinct stages of stomatal lineage,Citation4 but it remains unknown how cells interpret different signals using the same set of MAPKs to elicit specific responses during stomatal development. We recently identified an MKP1, which is one of the five Arabidopsis dual-specificity MKPs, that promotes the cell fate transition leading to stomatal differentiation.Citation5 By screening loss-of-function mutants of all five Arabidopsis MKPs, as well as an ARABIDOPSIS PROTEIN PHOSPHATASE2C (AP2C3) and a PROTEIN TYR PHOSPHATASE1 (PTP1) that are known to control activity of MPK3 and/or MPK6,Citation6–Citation8 we found that mkp1 in the Columbia (Col) ecotype exhibits an epidermis with stomatal development defects: a significantly reduced number of stomata with the formation of clusters of small cells.Citation5
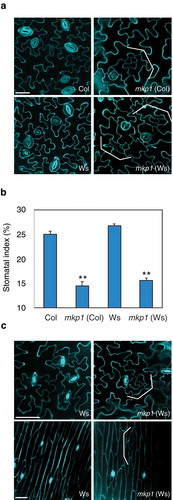
MKP1 is known as a key regulator of various environmental and biotic stress responses, and mkp1 mutant in the Wassilewskija (Ws) background, which is indistinguishable from the wild-type in standard condition, was used for studying its diverse functions in stress signaling pathways.Citation9 However, in contrast to mkp1 (Ws), it is reported that the mkp1 mutant in different background, Col, displays pleiotropic growth and developmental defects, including weak dwarfism and early senescence, and these phenotypes are known to associate with constitutive defense responses.Citation10 Therefore, we tested whether the stomatal defects of mkp1 (Col) found in our recent studies are also one of the ecotype-specific pleiotropic phenotypes. As shown in , we examined the epidermal phenotypes of mkp1 cotyledons in the Ws background, which do not have any growth phenotypes under normal growth conditions, and we observed that the epidermis of mkp1 (Ws) displays statistically fewer stomata (P < .001, Student's t test, ) with the formation of stomatal lineage ground cell (SLGC)-like clusters (brackets in ). These stomatal phenotypes of mkp1 (Ws) were also found in other mature organs, such as leaves and stems (). Taken together, our data clearly demonstrate that the epidermal defects of mkp1 are independent of the background accession, suggesting that MKP1 has a specific role in controlling stomatal development.
Figure 1. MKP1 is a positive regulator of stomatal development, independent of previously known ecotype-specific developmental defects.
(a) Representative confocal images of ten-day-old abaxial rosette leaf epidermis from wild-type (Col), mkp1 (Col), wild-type (Ws), and mkp1 (Ws). Both mkp1 (Col) and mkp1 (Ws) mutants exhibit similar stomatal development defects (reduction of stomata and clusters of small cells, indicated by brackets). Cells were outlined by propidium iodide staining (cyan), and images were taken under the same magnification. Scale bar = 30 µm. (b) Abaxial cotyledon stomatal index of ten-day-old seedlings, expressed as the percentage of the number of stomata to the total number of epidermal cells. n = 10 for each genotype. Bars, means. Error bars, s.e.m.**, P< .001 by Student’s t test. (c) Representative confocal images of five-week-old abaxial rosette leaf (top) and four-week-old stem epidermis (bottom) from wild-type Ws and mkp1 (Ws). Cells were outlined by propidium iodide staining (cyan), and images were taken under the same magnification. Scale bars, 50 μm.
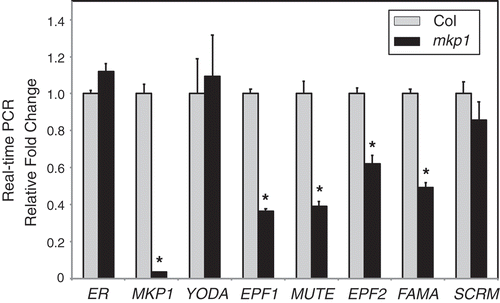
Time-course analyses of epidermal development using proTMM::GUS-GFP, which is expressed in stomatal-lineage cells, revealed the origin of the stomatal phenotypes of mkp1 (Col). The initial asymmetric divisions that produce stomatal lineage was indistinguishable in the wild-type Col and mkp1 (Col). However, meristemoids produced by asymmetric cell divisions were arrested and became pavement cells – as indicated by faint or no GFP signals as well as by the shape of cells – rather than being differentiated into stomata in the mkp1 (Col) epidermis.Citation5 To determine whether other known genes regulating stomatal development have a similar expression pattern as proTMM::GUS-GFP, we tested the expression levels of other stomatal regulators in mkp1 (Col). A qRT-PCR analysis using 10 dpg seedlings showed that expression levels of EPIDERMAL PATTERNING FACTOR1 (EPF1), EPF2, MUTE, and FAMA were reduced in mkp1 mutants (). Unlike these stomatal regulators, however, the expression level of the broadly expressed YODA was not changed in mkp1 seedlings compared with the wild-type Col (). TMM marks stomatal lineage cells, while EPF1, EPF2, MUTE, and FAMA mark a series of transitional states of stomatal precursors, starting from meristemoid mother cells to immature guard cells.Citation11–Citation14 Thus, altered expression of these stomatal regulators, which express in a series of distinct stomatal precursors, probably reflects the number of stomatal precursor cells in mkp1 (Col) compared to that of wild-type Col, and suggests MKP1’s function in promoting stomatal differentiation in the epidermis.
Figure 2. Transcript levels of stomata-related genes in mkp1(Col) mutants.
Ten-day-old seedlings were used for qRT-PCR analysis. Data were collected from three biological replicates, and the expression fold changes were normalized to the transcript level in wild-type (Col). Error bars represent standard deviations (n = 3). Significant difference compared with Col: *P< .01 by Student’s t test.
There is some evidence that phosphatases can be rapidly and transiently activated at the transcriptional and/or post-translational levels,Citation15–Citation18 and we found that no GFP signals driven by the MKP1 promoter were detected in spch epidermis,Citation5 indicating that the expression of MKP1 relies on SPCH. This result suggests the possibility of the presence of a new feedback loop for the specification of stomatal fate by activating MKP1, a negative regulator of stomatal MAPKs, MPK3 and MPK6, that inhibit stomatal differentiation. Here our qRT-PCR data showed that EPF2, which is expressed in early stomatal cell-types including meristemoid mother cells and meristemoids like SPCH expression, is reduced in mkp1 (Col). Thus, it is also possible that MKP1 can control initial asymmetric divisions regulated by SPCH to initiate stomatal lineage with other phosphatases although its main role is in regulating cell fate transition to stomata during stomatal development. On the basis of these findings, the precise regulation of MKP1 at the early stage of stomatal lineage could be critical for the specificity of MAPK signaling output during stomatal development. Therefore, further work is required to better understand the regulatory mechanisms of MKP1, which contributes MAPK signaling specificity and a proper biological response during stomatal development in plants.
Disclosure of Potential Conflict of Interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Komis G, Samajova O, Ovecka M, Samaj J. Cell and developmental biology of plant mitogen-activated protein kinases. Annu Rev Plant Biol. 2018;69:237–265. doi:10.1146/annurev-arplant-042817-040314.
- Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in arabidopsis. Plant Cell. 2007;19(1):63–73. doi:10.1105/tpc.106.048298.
- Bergmann DC, Lukowitz W, Somerville CR. Stomatal development and pattern controlled by a mapkk kinase. Science. 2004;304(5676):1494–1497. doi:10.1126/science.1096014.
- Lampard GR, Lukowitz W, Ellis BE, Bergmann DC. Novel and expanded roles for mapk signaling in arabidopsis stomatal cell fate revealed by cell type-specific manipulations. Plant Cell. 2009;21(11):3506–3517. doi:10.1105/tpc.109.070110.
- Tamnanloo F, Damen H, Jangra R, Lee JS. Map kinase phosphatase1 controls cell fate transition during stomatal development. Plant Physiol. 2018;178(1):247–257. doi:10.1104/pp.18.00475.
- Brock AK, Willmann R, Kolb D, Grefen L, Lajunen HM, Bethke G, Lee J, Nurnberger T, Gust AA. The arabidopsis mitogen-activated protein kinase phosphatase pp2c5 affects seed germination, stomatal aperture, and abscisic acid-inducible gene expression. Plant Physiol. 2010;153(3):1098–1111. doi:10.1104/pp.110.156109.
- Gupta R, Luan S. Redox control of protein tyrosine phosphatases and mitogen-activated protein kinases in plants. Plant Physiol. 2003;132:1149–1152.
- Bartels S, Gonzalez Besteiro MA, Lang D, Ulm R. Emerging functions for plant map kinase phosphatases. Trends Plant Sci. 2010;15(6):322–329. doi:10.1016/j.tplants.2010.04.003.
- Ulm R, Revenkova E, Di Sansebastiano GP, Bechtold N, Paszkowski J. Mitogen-activated protein kinase phosphatase is required for genotoxic stress relief in arabidopsis. Genes Dev. 2001;15(6):699–709. doi:10.1101/gad.192601.
- Bartels S, Anderson JC, Gonzalez Besteiro MA, Carreri A, Hirt H, Buchala A, Metraux JP, Peck SC, Ulm R. Map kinase phosphatase1 and protein tyrosine phosphatase1 are repressors of salicylic acid synthesis and snc1-mediated responses in arabidopsis. Plant Cell. 2009;21(9):2884–2897. doi:10.1105/tpc.109.067678.
- Hunt L, Gray JE. The signaling peptide epf2 controls asymmetric cell divisions during stomatal development. Curr Biol. 2009;19(10):864–869. doi:10.1016/j.cub.2009.03.069.
- Ohashi-Ito K, Bergmann DC. Arabidopsis fama controls the final proliferation/differentiation switch during stomatal development. Plant Cell. 2006;18(10):2493–2505. doi:10.1105/tpc.106.046136.
- Hara K, Kajita R, Torii KU, Bergmann DC, Kakimoto T. The secretory peptide gene epf1 enforces the stomatal one-cell-spacing rule. Genes Dev. 2007;21(14):1720–1725. doi:10.1101/gad.1550707.
- Pillitteri LJ, Sloan DB, Bogenschutz NL, Torii KU. Termination of asymmetric cell division and differentiation of stomata. Nature. 2007;445(7127):501–505. doi:10.1038/nature05467.
- Yamakawa H, Katou S, Seo S, Mitsuhara I, Kamada H, Ohashi Y. Plant mapk phosphatase interacts with calmodulins. J Biol Chem. 2004;279(2):928–936. doi:10.1074/jbc.M310277200.
- Zaidi I, Ebel C, Touzri M, Herzog E, Evrard JL, Schmit AC, Masmoudi K, Hanin M. Tmkp1 is a novel wheat stress responsive map kinase phosphatase localized in the nucleus. Plant Mol Biol. 2010;73(3):325–338. doi:10.1007/s11103-010-9617-4.
- Quettier AL, Bertrand C, Habricot Y, Miginiac E, Agnes C, Jeannette E, Maldiney R. The phs1-3 mutation in a putative dual-specificity protein tyrosine phosphatase gene provokes hypersensitive responses to abscisic acid in arabidopsis thaliana. Plant J. 2006;47(5):711–719. doi:10.1111/j.1365-313X.2006.02823.x.
- Park HC, Song EH, Nguyen XC, Lee K, Kim KE, Kim HS, Lee SM, Kim SH, Bae DW, Yun DJ, et al. Arabidopsis map kinase phosphatase 1 is phosphorylated and activated by its substrate atmpk6. Plant Cell Rep. 2011;30(8):1523–1531. doi:10.1007/s00299-011-1064-4.
