ABSTRACT
Indole-3-carboxylic acid (I3CA) is an indolic compound that induces resistance in Arabidopsis adult plants against the necrotrophic fungus Plectosphaerella cucumerina through primed callose accumulation. In this study, we confirm the relevance of ATL31 and SYP121 genes involved in vesicular trafficking in I3CA priming of defenses and we discard camalexin as a mediator of I3CA-induced resistance (IR) in adult plants. In addition, we observed that an intact I3CA biosynthetic pathway is necessary for I3CA-IR functionality.
Introduction
Plants are constantly being threatened by environmental challenges, such as abiotic and biotic stresses. Upon pathogenic challenge, they have developed a strong immune system adapted to fight back almost any kind of attacker by a complexity of coordinated defense responses.
Among the first layers of plant defense triggered by pathogens, papillae appositions containing callose are a rapid response occurring in the first stages of pathogen invasion. Callose is accumulated in the side of attack to strengthen the cell wall and prevent pathogen entry.Citation1
In Arabidopsis defense against Plectosphaerella cucumerina, tryptophan derived metabolites play also a key role. This is the case for indolic compounds and camalexin. Camalexin is an Arabidopsis phytoalexin known to be induced by a broad spectrum of pathogens. Among the indole-derivatives, I3CA levels were found to increase following a pathogenic infection.Citation2,Citation3,Citation4 I3CA was also found as a metabolite showing a priming profile upon P. cucumerina infection in β-aminobutyric acid (BABA)-treated Arabidopsis plants.Citation5 Later studies of our group showed that I3CA itself induces resistance in Arabidopsis against P. cucumerina and primes enhancement of callose accumulation.Citation6 Interestingly, we also found that I3CA induces the expression of the genes encoding two proteins involved in the vesicular transport of sugars to the membrane, SYP21 (PEN1) and its interactor ATL31, that were previously reported to regulate immune responsesCitation7 and suggested to be involved in the first stages of papillae formation.Citation8
The current study aims to support our previous work in Gamir et al. 2018Citation6 with complementary evidences of the involvement of vesicular trafficking in I3CA priming of callose upon P. cucumerina infection in Arabidopsis and to show new insights of the mechanisms of I3CA-IR.
Results and discussion
Vesicular trafficking proteins are key players in I3CA-IR
Plasma membrane-associated protein SYP121 and its interactor ATL31 gene expression levels were significantly increased by I3CA treatment in infected plants, showing a doubtless priming profile.Citation6 To further confirm these results in the present work we studied the response of mutants impaired in the expression of SYP121 and ATL31 against the infection of P. cucumerina in I3CA treated and untreated plants. Infection levels were quantified by a disease rating ().
Figure 1. Infection levels quantified by a disease rating in trypan blue stained leaves, measured as a percentage of infected leaf surface. Col-0 and mutant plants were treated with I3CA and infected with P.cucumerina. Colors mean % of diseased leaves in a scale (White-healthy leaves white; light grey-leaves with less than 20% of diseased surface; dark grey-leaves with more than 20% of the diseased surface, black-leaves with 100% of the surface diseases). Different letters indicate statistically significant differences (ANOVA, Fisher’s Least Significant Difference (LSD) test; P < .05 n = 15). The experiment was repeated three times with the same result.
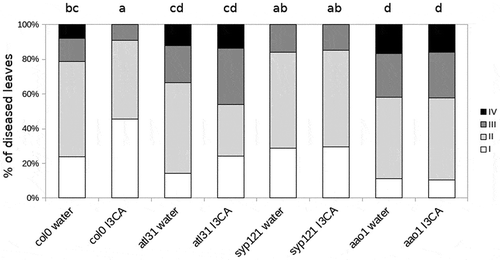
Both atl31 and syp121 mutants are impaired in I3CA-IR (). In addition, when both are treated with water display wild type infection levels (), suggesting that these proteins are key elements on the I3CA-IR but not in basal immunity. These observations place vesicular trafficking as an essential event in I3CA priming of defenses.
Intact I3CA biosynthetic pathway is necessary for I3CA-IR
Previous reports demonstrated that aldehyde oxidase1 (AAO1) gene is involved in the last step of I3CA biosynthesis.Citation2 In order to confirm that I3CA is indeed needed for the callose accumulation and responsible for the phenotype of induced resistance, a resistance bioassay was performed. The aao1 mutant was impaired in I3CA-IR. Furthermore, aao1 treated with water shows higher disease severity than the control (). This result suggests that an intact I3CA biosynthetic pathway is essential for I3CA-IR functionality as well as for basal resistance. Surprisingly, treatment of aao1 mutant with I3CA did not restore the wild type phenotype. Instead, it showed untreated-aao1 levels of infection ().
Measuring the I3CA levels by LC-MS we observed, on the one hand, that plants treated with I3CA did not have significantly higher amounts of this compound compared with control untreated plants when plants were not infected ()). The plant likely metabolizes the I3CA, using it as a source for the synthesis of other compounds. On the other hand, after the pathogen challenge plants increased the accumulation of I3CA and this accumulation was higher when plants were treated with I3CA ()). These results suggest that there is a positive loop or feedback in the regulation of its biosynthesis when the infection is present. This phenomenon would explain the previous phenotype observed in the aao1 mutant treated with I3CA. Since I3CA biosynthesis was suggested to be impaired in the mutantCitation2 the positive feedback on its biosynthesis and the additional increases in endogenous I3CA would not take place, hence the plant cannot be protected.
Figure 2. Effect of I3CA treatment and the infection in the accumulation of indole-derived compounds. Compounds levels were measured by HPLC-MS before and after Plectospharella cucumerina (Pc) infection in I3CA-treated (Col-I3CA) and untreated (Col-0) Arabidopsis Col-0 plants and are represented with box plots. (a) Indole-3-carboxylic acid (I3CA) levels. (b) Camalexin levels.
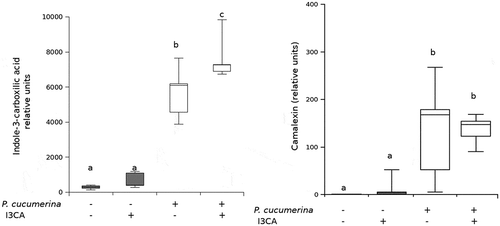
Camalexin is not responsive to I3CA-IR
To make new insights on the mechanisms of I3CA-IR against P.cucumerina we measured the levels of the phytoalexin camalexin in plants in the same conditions of the experiments mentioned above. As expected, camalexin levels increased significantly after the pathogen challenge in both control and treated plants ()). However, this increase was irrespective of the I3CA treatment ()) suggesting that camalexin is not a primed defense induced by I3CA treatment in adult plants, hence supporting the relevance of callose accumulation in I3CA-IR. Seemingly, Pastor et al. (2013)Citation9 did not find increases in camalexin following BABA treatments but induction of callose accumulation suggesting that effective early barriers of defense make no further necessary the activation of secondary defenses.
Acknowledgments
We thank the Serveis Centrals de la Universitat Jaume I for its technical support. This work was financially supported by the Plan de Promoción de la Investigación de la Universitat Jaume I UJI-B2016-43, MINECO AGL2015-64990-C2-2 and the Generalitat Valenciana AICO/2016/029.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest are disclosed.
Additional information
Funding
References
- Luna E, Pastor V, Robert J, Flors V, Mauch-Mani B, Ton J. Callose deposition: a multifaceted plant defense response. Mpmi. 2011;24:183–193. doi:10.1094/MPMI-07-10-0149.
- Böttcher C, Chapman A, Fellermeier F, Choudhary MR, Scheel D, Glawischnig E. The biosynthetic pathway of indole-3-carbaldehyde and indole-3-carboxylic acid derivatives in arabidopsis. Plant Physiol. 2014;165:841–853. doi:10.1104/pp.114.235630.
- Gamir J (a), Pastor V, Kaever A, Cerezo M, Flors V. Targeting novel chemical and constitutive primed metabolites against Plectosphaerella cucumerina. Plant J. 2014;78:227–240. doi:10.1111/tpj.12465.
- Gamir J (b), Sanchez-Bel P, Flors V. Molecular and physiological stages of priming: how plants prepare for environmental challenges. Plant Cell Rep. 2014;33:1935–1949. doi:10.1007/s00299-014-1665-9.
- Gamir J, Pastor V, Cerezo M, Flors V. Identification of indole-3-carboxylic acid as mediator of priming against Plectosphaerella cucumerina. Plant Physiol Biochem. 2012;61:169–179. doi:10.1016/j.plaphy.2012.10.004.
- Gamir J, Pastor V, Sánchez-Bel P, Agut B, Mateu D, garcía-Andrade J, Flors V. Starch degradation, abscisic acid and vesicular trafficking are important elements in callose priming by indole‐3‐carboxylic acid in response to Plectosphaerella cucumerina infection. Plant J. 2018;96:518–531. doi:10.1111/tpj.14045.
- Maekawa S, Sato T, Asada Y, Yasuda S, Yoshida M, Chiba Y, Yamaguchi J. The Arabidopsis ubiquitin ligases ATL31 and ATL6 control the defense response as well as the carbon/nitrogen response. Plant Mol Biol. 2014;79:217–227. doi:10.1007/s11103-012-9907-0.
- Assaad FF, Qiu JL, Youngs H, Ehrhardt D, Zimmerli L, Kalde M, Wanner G, Peck SC, Edwards H, Ramonell K, et al. The PEN1 syntaxin defines a novel cellular compartment upon fungal attack and is required for the timely assembly of papillae. Mol Biol Cell. 2004;15:5118–5129. doi:10.1091/mbc.E04-02-0140.
- Pastor V, Luna E, Ton J, Cerezo M, García-Agustín P, Flors V. Fine tuning of reactive oxygen species homeostasis regulates primed immune responses in Arabidopsis. Mol Plant Microbe Interact. 2013;26: 1334-44. doi:10.1094/MPMI-04-13-0117-R.
