ABSTRACT
Plants use many natural products to counter pests and diseases in nature. In rice, direct defense mechanisms include broad range of secondary metabolites, such as phenolamides (PA), diterpene phytoalexins, and flavonoid sakuranetin. Recently, accumulation of PAs in rice was shown to be under control of microbial symbionts in honeydew (HD), digestive waste from the rice brown planthopper (Nilaparvata lugens; BPH), but whether HD microbiota can also promote diterpene phytoalexins, momilactone A (MoA) and MoB, has not been reported. Here, we demonstrate that crude HD, but not a filtered one, induces MoA and MoB in rice, suggesting the involvement of BPH-HD endosymbionts. Consequently, microbial strains previously isolated from HD could promote MoA and MoB levels in wounded rice leaves, suggesting that rice indeed responds to BPH by cumulative chemical defense that involves both PA and diterpene phytoalexin pathways.
Symbiosis between insect pests and microbes has been known for a long time. For instance, Rickettsia sp. in Bemisia tabaci,Citation1 Sporothrix sp. in Bursaphelenchus xylophilus,Citation2 Leptographium procerum in Dendroctonus valens,Citation3 Microsporidia in Harmonia axyridis,Citation4 Streptomyces, γ-Proteobacteria and Amylostereum areolatum in Sirex noctilio,Citation5 and Raffaelea lauricola in Xyleborus glabratusCitation6 are just few examples to name with mutual relations. Close partnership between microbes and insects often negatively affects host plants, such as tomato yellow leaf curly virus (TYLCV) and tomato chlorosis virus (ToCV) in B. tabaci that simultaneously damage tomato plants.Citation1 On the other hand, plants evolved whole arsenal of natural products to counter pests and pathogens, and other competitive factors.Citation7 For example, Arabidopsis thaliana accumulates camalexins and sesquiterpenes, and oat (Avena sativa) activates triterpenoid saponins as plant defense products against herbivores and/or pathogens.Citation8 Rice (Oryza sativa) similarly quickly and universally accumulates phenolamides (PAs), p-coumaroylputrescine (CoP), and feruloylputrescine (FP) in response to attack by both chewing insects, i.e. feeding of the lawn armyworm (Spodoptera mauritia) and the rice skipper (Parnara guttata) larvae, and the attack of the sucking insect, the rice brown planthopper (Nilaparvata lugens, BPH).Citation9 Momilactones (diterpenes) are another front defense chemicals in rice that are induced by fungal blast agent Magnaporthe oryzae,Citation10 attack of sucking insect pests such as BPH,Citation9 but not by mechanical wounding, suggesting their broad but not well-understood role in rice defense against multiple biotic stresses.
In particular, momilactone A (MoA) and MoB work as antimicrobial defense compounds,Citation11 and rice plants secrete MoA and MoB from their roots into the rhizosphere where these allelopathics inhibit the growth of competing weeds.Citation12 Variable levels of MoA and MoB were induced by different feeding guilds of insects,Citation9 with BPH being the most effective inducer. Interestingly, BPH excretes sugar-rich digestive waste, honeydew (HD) on plants that supports microbial growth, and causes sooty appearances of rice leaves in paddy fields during heavy BPH seasons. It has been recently shown that HD contains symbiotic microbesCitation13,Citation14 that are recognized by rice defense systems and trigger rice defense metabolism, as it was recently exemplified on PA class compounds in Wari et al.Citation14 Here, we assess if HD-associated microbes could also affect momilactone levels that would explain their differential accumulation in response to BPH, while remaining unchanged in case of mechanical wounding.
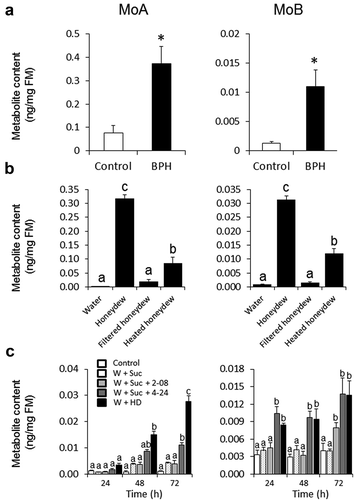
Following protocols developed in our previous study,Citation14 we first confirmed the accumulation of MoA and MoB in response to BPH feeding, using analytical methods for MoA and MoB described in Alamgir et al.Citation9 As expected, BPHs (n = 10) attached to last fully expanded young leaf of 6-week-old rice seedlings, enclosed in ventilated cages for 4 d, significantly elevated MoA and MoB levels in the leaves (). Next, crude HD (2 μL) was gently rubbed on the leaf surface of the same-age leaves as before which clearly elicited MoA and MoB levels at 72 h, as shown in . We then used size-fractionated and heat-treated HD to characterize dominant elicitor that is required for momilactone induction. As elicitor activity was removable by 0.22 µm filter, and partially heat tolerant, dominant elicitor could be narrowed down to yet unknown component associated with microbial cells (). We then used our previously isolated symbionts from HD and test them for MoA and MoB induction with young rice leaves.Citation14 Pattern wheel wounding on each side of leaf lamina was introduced prior to rubbing of microbes suspended in 15% sucrose (2 μL) to facilitate their easier penetration into rice cells. Previously shown as strongest inducer of PAs,Citation14 symbiont 4–24 (Serratia marcescens subsp. marcescens) applied in 15% sugar also significantly induced MoA and MoB levels compared to sucrose-treated (mock) leaves. These levels were comparable to crude HD treatment on wounded leaves. As before,Citation14 strain 2–08 was much less effective in inducing MoA and MoB levels (). Corroborating the importance of BPH HD-associated symbionts for elicitation of plant defense, our data indicate that, in addition to PAs and volatile organic compounds,Citation14 rice cells also activate diterpene-based defense upon receiving HD information encoded by its inhabitants, facultative and/or obligate endosymbionts from BPH.
Figure 1. Accumulation of momilactones A and B in rice leaves. (A) Metabolite content measured in rice leaves by LC-MS/MS after 96 h infestation with BPH. Asterisks indicate statistically significant differences between treatment and control determined by Student’s t-test (*P < .05). n = 5–8; error bars = SEM; FM, fresh mass. (B) Momilactone A and B contents in rice leaves treated with raw or processed HD (filtered, 0.22 μM; or heat treated, 100°C, 20 min) determined by LC-MS/MS after 72 h treatment. Different letters show statistically significant differences between treatments by ANOVA (P < .05; Tukey HSD test). n = 3; error bars = SEM; FM, fresh mass; (C) Momilactone A and B contents in leaves after wounding with a serrated pattern wheel and applying microbial isolates from HDCitation14 suspended in 15% (w/v) sucrose adjusted to OD600 = 0.2. Metabolite levels were determined every 24 h after treatment by LC-MS/MS. Sucrose and HD were used as negative and positive controls, respectively. Different letters show statistically significant differences between treatments at the same time point determined by ANOVA (P < .05; Tukey HSD test). n = 4; error bars = SEM; W+ Suc, wounding with 15% sucrose; W+ Suc+2–08, wounding with isolate 2–08 suspended in 15% sucrose; W+ Suc+4–24, wounding with isolate 4–24 suspended in 15% sucrose; W+ HD, wounding with raw BPH honeydew; W, wounding; Suc, sucrose; HD, honeydew; FM, fresh mass.
In rice, multiple terpene synthase genes are induced by environmental factors that result in accumulation of terpenoid phytoalexins, momilactones, and phytocassanes.Citation15 A number of transcription factors (TFs) have already been characterized as regulators of biosynthesis of rice terpenoids, and these factors are then likely to connect to the perception of BPH symbionts in rice.Citation16 As sucking insect attack is often described as bearing pathogen signatures, it may suggest that due to a lack of mechanical damage as leading signal of attack, plants adopted less typical but reliable signals from the enemy to detect them. It is also possible to speculate that some of the antimicrobial signaling pathways became newly cross-linked to those for anti-herbivore defenses, or that plants have adopted broadly toxic chemicals as multifaceted unified weapons against variable biotic stress factors. It will be interesting to examine further plant defense pathways and crosstalk between pathogen (salicylic acid) and herbivore (jasmonate) signaling in plants that is usually considered negative in case of chewing insects but it can be perceived as more positive in case of sucking insect attack.
Author Contributions
D.W. designed and conducted experiments, analyzed data, wrote paper. K.M.A. designed and conducted experiments. K.M., Y.H., and H.N. conducted and assisted with experiments; T.S. conducted experiments, wrote paper. A.T. conducted experiments, wrote paper. I.G. designed, supervised and conducted experiments, and wrote paper.
Discloser of Potential Conflict of Interest
The authors report no conflict of interest.
Acknowledgments
We thank Dr. Masaya Matsumura (Kyushu Okinawa Agricultural Research Center, NARO) for providing field-collected BPHs; Ms. Yoshiko Fujitani for technical assistance during microbe isolations; Dr. Kazunori Okada (Tokyo University) for providing purified standards of momilactone A and B.
Additional information
Funding
References
- Himler AG, Adachi-Hagimori T, Bergen JE, Kozuch A, Kelly SE, Tabashnik BE, Chiel E, Duckworth VE, Dennehy TJ, Zchori-Fein E, et al. Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science. 2011;332:254–265. doi:10.1126/science.1199410.
- Zhao L, Lu M, Niu H, Fang G, Zhang S, Sun J. A native fungal symbiont facilitates the prevalence and development of an invasive pathogen-native vector symbiosis. Ecology. 2013;94:2817–2826. doi:10.1890/12-2229.1.
- Lu M, Wingfield MJ, Gillette NE, Mori SR, Sun JH. Complex interactions among host pines and fungi vectored by an invasive bark beetle. New Phytol. 2010;187:859–866. doi:10.1111/j.1469-8137.2010.03316.x.
- Vilcinskas A, Stoecker K, Schmidtberg H, Röhrich CR, Vogel H. Invasive harlequin ladybird carries biological weapons against native competitors. Science. 2013;340:862–863. doi:10.1126/science.1234032.
- Talbot PHB. The Sirex-Amylostereum-Pinus association. Annu Rev Phytopathol. 1977;15:41–54. doi:10.1146/annurev.py.15.090177.000353.
- Fraedrich SW, Harrington TC, Rabaglia RJ, Ulyshen MD, Mayfield AE, Hanula JL, Eickwort JM, Miller DR. A fungal symbiont of the redbay ambrosia beetle causes a lethal wilt in redbay and other Lauraceae in the southeastern United States. Plant Dis. 2008;92:215–224. doi:10.1094/PDIS-92-2-0215.
- Bednarek P, Osbourn A. Plant-microbe interactions: chemical diversity in plant defense. Science. 2009;324:746–748. doi:10.1126/science.1171661.
- Papadopoulou K, Melton RE, Leggett M, Daniels MJ, Osbourn AE. Compromised disease resistance in saponin-deficient plants. Proc Natl Acad Sci USA. 1999;96:12923–12928. doi:10.1073/pnas.96.22.12923.
- Alamgir KM, Hojo Y, Christeller JT, Fukumoto K, Isshiki R, Shinya T, Galis I. Systematic analysis of rice (Oryza sativa) metabolic responses to herbivory. Plant Cell Environ. 2016;39:453–466. doi:10.1111/pce.12640.
- Cartwright DW, Langcake P, Pryce RJ, Leworthy DP, Ride JP. Isolation and characterization of two phytoalexins from rice as momilactones A and B. Phytochem. 1981;20:535–537. doi:10.1016/S0031-9422(00)84189-8.
- Jung YH, Lee JH, Agrawal GK, Rakwal R, Kim JA, Shim JK, Lee SK, Jeon JS, Koh HJ, Lee YH, et al. The rice (Oryza sativa) blast lesion mimic mutant, blm, may confer resistance to blast pathogens by triggering multiple defense-associated signaling pathways. Plant Physiol Biochem. 2005;43:397–406. doi:10.1016/j.plaphy.2005.03.002.
- Kato-Noguchi H, Ino T, Sata N, Yamamura S. Isolation and identification of a potent allelopathic substance in rice root exudates. Physiol Plant. 2002;115:401–405. doi:10.1034/j.1399-3054.2002.1150310.x.
- Wang W, Zhu T, Lai F, Fu Q. Diversity and infection frequency of symbiotic bacteria in different populations of the rice brown planthopper in China. J Entomol Sci. 2015;50:47–66. doi:10.18474/0749-8004-50.1.47.
- Wari D, Kabir MA, Mujiono K, Hojo Y, Shinya T, Tani A, Nakatani H, Galis I. Honeydew-associated microbes elicit defense responses against brown planthopper in rice. J Exp Bot. 2019;70:1683–1696. doi:10.1093/jxb/erz041.
- Hasegawa M, Mitsuhara I, Seo S, Imai T, Koga J, Okada K, Yamane H, Ohashi Y. Phytoalexin accumulation in the interaction between rice and the blast fungus. Mol Plant Microbe Interact. 2010;23:1000–1011. doi:10.1094/MPMI-23-8-1000.
- Fukushima S, Mori M, Sugano S, Takatsuji H. Transcription factor WRKY62 plays a role in pathogen defense and hypoxia-responsive gene expression in rice. Plant & Cell Physiol. 2016;57:2541–2551. doi:10.1093/pcp/pcw185.
