ABSTRACT
Singlet oxygen (1O2) is a potent oxidizing agent, principally generated by photosystem II (PSII) as a byproduct of photosynthesis. Hence, 1O2 damages PSII, especially the PSII reaction center (RC) proteins, promoting a process called PSII repair cycle. The hetero-hexameric FtsH protease, located in the thylakoid membrane, is essential in degrading these damaged PSII RC proteins, which defines the first step of the PSII repair. The loss of the central subunit of the FtsH protease, FtsH2 (VAR2), weakens the PSII repair, thereby impairing PSII proteostasis. A recent study demonstrated that the impaired proteostasis (or accumulation of damaged proteins) in the chloroplasts of the var2 mutant induces an unfolded/misfolded protein response (UPR)-like response, more appropriately referred to as a damaged protein response (DPR), as evident in the accumulation of proteins related to the protein quality control (PQC). Comparison of data from chloroplast proteomics data with RNA sequencing in the context of the UPR-like response suggests a plausible activation of retrograde signaling in the var2 mutant. Either through the enhanced level of 1O2 or by impairing the substrate-unfolding activity of FtsH2, the reinforced defect appears to induce stress-related genes via the stress hormone salicylic acid (SA). This finding suggests that impaired chloroplast proteostasis (specifically for PSII proteins) may activate the chloroplast-established isochorismate pathway to produce SA. If this assumption is correct, then SA serves as a retrograde signaling molecule. In this review, we will discuss the impact of chloroplast proteostasis on chloroplasts-to-nucleus communication.
Introduction
The chloroplast is a central organelle sensing environmental fluctuations. Upon perception of stimuli, chloroplasts drive retrograde signaling (RS) pathways to communicate with the nucleus.Citation1–Citation3 Multiple lines of evidence demonstrate that such communication confers plant resistance to various environmental stimuli. A decade of studies on RS has identified chloroplast-generated signaling molecules contributing to plant stress responses such as acclimation and cell death.Citation4–Citation8 These signaling molecules include reactive oxygen species (ROS), reactive electrophile species (RES), a phosphonucleotide, and a precursor of isoprenoids.Citation6,Citation9,Citation10 Besides, chloroplasts play an indispensable role in producing stress-related hormones such as salicylic acid (SA), jasmonic acid (JA), and abscisic acid (ABA).Citation11–Citation13
Figure 1. Chloroplast proteostasis-associated retrograde signaling pathways. Inactivation of FtsH2 results in the accumulation of damaged proteins in the chloroplasts of var2-ko. This defect causes enhanced levels of ROS and a change in redox status in the chloroplasts, further impairing the overall proteostasis. The dysfunctional chloroplasts then readjust chloroplast proteostasis, most likely via RS, leading to the accumulation of proteins involved in PQC and ROS detoxification. The reinforced problem in proteostasis appears to also increase the cellular SA content via the chloroplast ICS pathway in the absence of transcriptional induction of genes involved in SA synthesis. SA then rapidly induces SRGs.
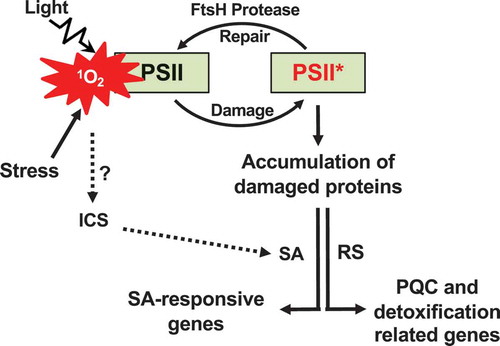
Abiotic and biotic stress enhance ROS levels in chloroplasts, which spontaneously changes the redox status. Among these ROS, singlet oxygen (1O2) is found to trigger distinct RS pathways. The spatially and functionally separated 1O2 sensors β-carotene and the EXECUTER1 (EX1) proteins mediate these RS pathways.Citation6,Citation14,Citation15 Besides its role in RS, 1O2 is a prime ROS damaging photosystem II (PSII) reaction center (RS) proteins (such as D1) owing to their vicinity to the side of 1O2 production.Citation16–Citation18 This damaged PSII RC undergoes a repair process in the non-appressed regions of the thylakoids. The membrane-bound FtsH protease degrades the damaged PSII RC proteins, facilitating the reassembly of PSII. Besides the PSII repair and proteostasis, the FtsH protease coordinates EX1-mediated 1O2 signaling by promoting EX1 proteolysis.Citation14,Citation19 Thus, loss of FtsH protease impairs both PSII repair (PSII proteostasis) and EX1-mediated 1O2 signaling. Despite the importance of PSII, an Arabidopsis var2 null (var2-ko) mutant lacking functional FtsH protease is viable.Citation16,Citation20,Citation21 This suggests that dysfunctional chloroplasts of var2-ko may reprogram proteostasis via RS. Consistently, a recent study on var2 revealed that chloroplasts deficient in PSII proteostasis induce an RS to activate a stress response which resembles the unfolded/misfolded protein response (UPR).Citation21 Moreover, the reinforced PSII proteostasis and the accumulation of damaged proteins appear to promote SA synthesis via the chloroplast-established isochorismate (ICS) pathway.Citation22 This finding suggests a potential interconnection between the impaired proteostasis (or its related problem such as altered redox status) and the stimulation of the ICS pathway.
The role of FtsH protease EX1-mediated 1O2 signaling
The identification of the Arabidopsis fluorescent (flu) mutant, which conditionally generates 1O2 in chloroplasts upon a dark-to-light shift, enables to establish the signaling role of 1O2. The FLU protein serves as a negative regulator of tetrapyrrole biosynthesis in the dark. The overaccumulation of protochlorophyllide (Pchlide) in the dark implies a role of FLU in the Mg-branch instead of the Fe-branch.Citation23 The excess amounts of Pchlide result in the burst of 1O2 upon illumination as free Pchlide acts as a potent photosensitizer. It appears that 1O2 induces stress responses (cell death and growth inhibition) via a genetic program rather than resulting from the cytotoxicity of 1O2 in flu.Citation4 The forward genetic screen identified the nuclear-encoded chloroplast EX1 protein as being responsible for initiating 1O2 signaling. A recent study by Wang et al. shed light on the possible mode of action of EX1 in mediating 1O2 signaling.Citation14 EX1 seems to almost exclusively localize in the non-appressed regions of the grana (the grana margin) and associates with PSII RC proteins, chlorophyll biosynthesis enzymes, translational machinery, and the FtsH protease.Citation14 Upon 1O2 generation, EX1 undergoes an FtsH-dependent proteolysis, which is the prerequisite for initiating RS.Citation14,Citation19 Furthermore, EX1 likely senses 1O2 through physical interaction, leading to the oxidative post-translational modification at a specific Tryptophan (Trp) residue in the 1O2 sensor (SOS) domain located at the C-terminus of EX1.Citation15 This modification may facilitate its degradation by FtsH protease, thereby initiating 1O2 signaling.
Loss of FtsH2 induces a damaged protein response
PSII produces 1O2 as a byproduct of photosynthesis, which in return becomes a prime target of 1O2. Although β-carotene molecules present in PSII scavenge 1O2, due to the imminent reactivity of 1O2, PSII damage is inevitable.Citation24,Citation25 Thus, 1O2 generation and PSII damage are considered as default processes during photosynthesis, which tend to increase under photo-oxidative stress conditions.Citation21,Citation22,Citation26 The damaged PSII continually undergoes a repair process at the non-appressed regions of the thylakoids. The degradation of the damaged PSII RC proteins such as D1 by the FtsH protease is required to promote PSII reassembly. Under extreme photoinhibitory conditions, the rate of 1O2 generation exceeds the scavenging capacity leading to excessive damage to the PSII RC and the repair machinery (such as the FtsH protease).Citation27 This extensive damage eventually impairs the PSII repair process, which then alters the overall chloroplast proteostasis. Collectively, we infer that FtsH protease is involved in 1O2 signaling and chloroplast proteostasis [also can be referred to as protein quality control (PQC)].Citation21,Citation28 In plants, the FtsH protease is present as a hetero-hexamer constituted by four subunits of two major isoforms: Type A, including FtsH1 and FtsH5 (called VAR1), and Type B, including FtsH2 (called VAR2) and FtsH8.Citation17,Citation29 Among these subunits, inactivation of either FtsH2 or FtsH5 fails the PSII repair, resulting in the failure of light acclimation and the emergence of leaf variegation.Citation17,Citation29 Given that PSII repair is a default process and that both var1 and var2 null mutants are viable under moderate light conditions, it is conceivable that these mutants may reprogram chloroplast proteostasis to minimize chloroplast dysfunction. Comparative chloroplast proteome analysis in var2-ko and WT plants grown under moderate continuous light conditions revealed a heightened expression of chloroplast PQC components including chaperones, proteases, and detoxification-related proteins in var2-ko.Citation21 Interestingly, this molecular signature (i.e., upregulation of PQC components) resembles the unfolded/misfolded protein response (UPR), which is prompted upon endoplasmic reticulum (ER) stress.Citation30
Besides, since the photosynthetic apparatus is the primary site of ROS generation and as an adaptive measure, chloroplasts of var2-ko mutant show substantial repression in the accumulation of both nuclear- as well as plastid-encoded photosynthetic components. Remarkably, even though the majority of photosynthetic proteins are down-regulated, the PSII core proteins D1, D2, CP43, and CP47 are markedly accumulated in var2-ko. Perhaps, not only D1 but also all these D1-associated proteins undergo degradation by the FtsH protease. Although D1 is believed to undergo 1O2-dependent oxidation, the specific sites of this oxidation were unclear until recently. Interestingly the study which revealed the 1O2-dependent oxidative modification in EX1 (Trp-643 residue in the SOS domain), also found the oxidations at specific Trp residues in EX1-associated PSII core proteins.Citation15 Consistently, a comprehensive chloroplast proteome analysis via mass spectrometry (MS) analysis also revealed the same Trp oxidations in PSII core proteins in the WT and var2-ko plants grown under normal light conditions, coinciding with the previous report showing a plausible link between Trp oxidations and PSII damage.Citation31,Citation32 This observation supports the notion that PSII undergoes continuous damage by 1O2, regardless of light intensity.Citation26 Besides, it also provides direct experimental evidence showing the accumulation of damaged proteins due to impaired PSII repair. The MS analysis also revealed Trp oxidations in other photosynthesis-associated proteins such as PSI RC proteins. Considering their increased accumulation in var2-ko, it is conceivable that these proteins can be putative substrates of the FtsH protease. Given the accumulation of damaged proteins by ROS, the UPR-like response can be more precisely referred to as a damaged protein response (DPR) in var2-ko.
In addition to its oxidation, the D1 protein also undergoes phosphorylation via State Transition Kinase 8 (STN8), which is thought to facilitate the migration of damaged PSII to the grana margin for repair.Citation33 Therefore, the extent of phosphorylation of the D1 protein is often regarded as the degree of photodamage of PSII. After knowing the Trp oxidation sites, MS analysis now enables us to accurately quantify the level of PSII damage. However, given the sophistication and expensiveness of the MS analysis, the development of a cost-effective and easy-to-perform biochemical detection method for Trp-oxidation would be a useful tool to further study photo-oxidative damage.
Impaired proteostasis and salicylic acid (SA)-mediates RS
Since the vast majority of chloroplast proteins are nuclear-encoded, chloroplasts continue their communication with the nucleus, enabling chloroplastic homeostasis.Citation1,Citation2 For instance, RS seems to cause the up-regulation of nuclear-encoded plastid proteins involved in PQC and detoxification in var2-ko. Likewise, the downregulation of nuclear-encoded photosynthesis-associated proteins correlates with their cognate transcript levels.Citation21 The equivalent transcriptome and chloroplast proteome levels are also observed in an additional var2 allele, namely var2-9. The lack of PSII repair is similar to that of the var2 null mutant, but var2-9 expresses a mutant FtsH2 protein deficient in the substrate-unfolding activity. The presence of abnormal FtsH2 in var2-9 leads to a relatively higher accumulation of damaged PSII core proteins relative to the var2-ko mutant.Citation22
This difference is seemingly more visible in nuclear gene expression changes: as compared to the var2-ko mutant, a subset of SA-responsive genes (SRGs) are significantly upregulated in var2-9.Citation22 Given that chloroplasts highly demand PSII repair under a heightened light intensity, it is rational to expect the higher accumulation of damaged PSII proteins in var2-ko mutant grown under higher light intensity. If the increased accumulation of photodamaged proteins in var2-9 relative to var2-ko is a cause of the expression of SRGs, then increased light intensity should induce SRGs in var2-ko but not in wild-type plants. Indeed, a rapid upregulation of SRGs was observed in var2-ko mutant plants, but not in wild-type plants, upon exposure to moderately increased light intensity.Citation22 Nevertheless, either inactivation of SA signaling components [such as Enhanced Disease Susceptibility 3 (EDS3) and Phytoalexin Deficient 4 (PAD4)] or depletion of SA (by expressing the bacterial SA hydrolyzing enzyme NahG) abrogates the expression of SRGs. Furthermore, the subtle amount of SA accumulation (around 1.5 fold higher than in wild-type plants) is sufficient to induce the expression of SRGs in var2-9.Citation22 The uncoupled accumulation of SA to the transcripts involved in SA biosynthesis suggests that the chloroplast ICS pathway may be activated due to the impaired PSII repair (or proteostasis) (). If this hypothesis is proven, this finding opens a new research avenue with SA acting as a retrograde signaling molecule.
Conclusions and prospects
A recent study on the yellow leaf variegation mutant var2-ko revealed that impaired PSII proteostasis results in a rapid induction of a suite of genes encoding proteins involved in PQC and detoxification after the onset of the photosynthesis (Figure 1). In this review, we dubbed this pathway as DPR instead of UPR-like response, as evident in an increase of ROS and the accumulation of damaged (oxidized) proteins in the var2 mutant.Citation21 At present, it is uncertain whether this reprogramming plays an essential role in sustaining var2-ko mutant plants under normal growth conditions despite the impaired PSII repair. Further research, i.e. the loss or overexpression of the PQC- and detoxification-related genes in var2-ko, would thus provide a clue towards the importance of such transcriptional reprogramming via RS (). This finding also appeals a further study on dissecting the DPR pathway on how the dysfunctional chloroplasts initiate the DPR pathway. Forward and reverse genetic approaches may enable us to dissect and to find the underlying mechanism of this pathway.
The reinforced lesion in chloroplast proteostasis seems to induce SRGs via SA mostly synthesized through the chloroplast ICS pathway.Citation22 This observation suggests a possible connection between chloroplast proteostasis and SA-mediated plant stress responses including plant immunity. However, the link between impaired PSII proteostasis and activation of the ICS pathway needs to be elucidated (). Further investigations on the role of PSII repair (or proteostasis) in plants upon pathogen attack would provide an insight into the role of chloroplasts in plant immune responses.
Disclosure of Potential Conflicts of Interest
There is no potential conflict of interest.
Acknowledgments
This work was supported by the Strategic Priority Research Program from the Chinese Academy of Sciences (Grant No. XDB27040102). The supports from Research Fund for International Young Scientists Program of NSFC (Grant No. 31850410478) and President’s International Fellowship Initiative (PIFI) postdoctoral fellowship from the Chinese Academy of Sciences (No. 2019PB0066) to V.D. are also acknowledged.
Additional information
Funding
References
- Chan KX, Phua SY, Crisp P, McQuinn R, Pogson BJ. Learning the languages of the chloroplast: retrograde signaling and beyond. Annu Rev Plant Biol. 2016;67:25–53. doi:10.1146/annurev-arplant-043015-111854.
- Woodson JD, Chory J. Organelle signaling: how stressed chloroplasts communicate with the nucleus. Curr Biol. 2012;22:R690–2.
- Kleine T, Voigt C, Leister D. Plastid signalling to the nucleus: messengers still lost in the mists?. Trends Genet. 2009;25:185–192.
- Op Den Camp RG, Przybyla D, Ochsenbein C, Laloi C, Kim C, Danon A, Wagner D, Hideg E, Göbel C, Feussner I, et al. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell. 2003;15:2320–2332.
- Kim C, Meskauskiene R, Zhang S, Lee KP, Lakshmanan Ashok M, Blajecka K, Herrfurth C, Feussner I, Apel K. Chloroplasts of Arabidopsis are the source and a primary target of a plant-specific programmed cell death signaling pathway. Plant Cell. 2012;24:3026–3039.
- Ramel F, Birtic S, Ginies C, Soubigou-Taconnat L, Triantaphylides C, Havaux M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc Natl Acad Sci U S A. 2012;109:5535–5540.
- Fischer BB, Krieger-Liszkay A, Hideg É, Šnyrychová I, Wiesendanger M, Eggen RIL. Role of singlet oxygen in chloroplast to nucleus retrograde signaling in Chlamydomonas reinhardtii. FEBS Letters. 2007;581:5555–5560.
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399.
- Estavillo GM, Crisp PA, Pornsiriwong W, Wirtz M, Collinge D, Carrie C, Giraud E, Whelan J, David P, Javot H, et al. Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell. 2011;23:3992–4012.
- Xiao Y, Savchenko T, Baidoo EE, Chehab WE, Hayden DM, Tolstikov V, Corwin JA, Kliebenstein DJ, Keasling JD, Dehesh K. Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell. 2012;149:1525–1535.
- Chen Z, Zheng Z, Huang J, Lai Z, Fan B. Biosynthesis of salicylic acid in plants. Plant Signal Behav. 2009;4:493–496.
- Finkelstein R. Abscisic Acid synthesis and response. Arabidopsis Book. 2013;11:e0166.
- Turner JG, Ellis C, Devoto A. The jasmonate signal pathway. Plant Cell. 2002;14:S153–64.
- Wang L, Kim C, Xu X, Piskurewicz U, Dogra V, Singh S, Mahler H, Apel K. Singlet oxygen- and EXECUTER1-mediated signaling is initiated in grana margins and depends on the protease FtsH2. Proc Natl Acad Sci U S A. 2016;113:E3792–800.
- Dogra V, Li M, Singh S, Li M, Kim C. Oxidative post-translational modification of EXECUTER1 is required for singlet oxygen sensing in plastids. Nat Commun. 2019;10:2834.
- Kato Y, Miura E, Ido K, Ifuku K, Sakamoto W. The variegated mutants lacking chloroplastic FtsHs are defective in D1 degradation and accumulate reactive oxygen species. Plant Physiol. 2009;151:1790–1801.
- Sakamoto W, Zaltsman A, Adam Z, Takahashi Y. Coordinated regulation and complex formation of yellow variegated1 and yellow variegated2, chloroplastic FtsH metalloproteases involved in the repair cycle of photosystem II in Arabidopsis thylakoid membranes. Plant Cell. 2003;15:2843–2855.
- Aro EM, McCaffery S, Anderson JM. Photoinhibition and D1 protein degradation in peas acclimated to different growth irradiances. Plant Physiol. 1993;103:835–843.
- Dogra V, Duan J, Lee KP, Lv S, Liu R, Kim C. FtsH2-dependent proteolysis of executer1 is essential in mediating singlet oxygen-triggered retrograde signaling in arabidopsis thaliana. Front Plant Sci. 2017;8:1145.
- Kato Y, Sakamoto W. FtsH protease in the thylakoid membrane: physiological functions and the regulation of protease activity. Front Plant Sci. 2018;9:855.
- Dogra V, Duan J, Lee KP, Kim C. Impaired PSII proteostasis triggers a UPR-like response in the var2 mutant of Arabidopsis. J Exp Bot. 2019;70:3075–3088.
- Duan J, Lee KP, Dogra V, Zhang S, Liu K, Caceres-Moreno C, Lv S, Xing W, Kato Y, Sakamoto W, et al. Impaired PSII proteostasis promotes retrograde signaling via salicylic acid. Plant Physiol. 2019;180:2182–2197.
- Meskauskiene R, Nater M, Goslings D, Kessler F, Op Den Camp R, Apel K. FLU: a negative regulator of chlorophyll biosynthesis in arabidopsis thaliana. Proc Natl Acad Sci U S A. 2001;98:12826–12831.
- Triantaphylides C, Krischke M, Hoeberichts FA, Ksas B, Gresser G, Havaux M, Van Breusegem F, Mueller MJ.Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol. 2008;148:960–968.
- Kato Y, Sakamoto W. Protein Quality Control in Chloroplasts: A current model of D1 protein degradation in the photosystem II repair cycle. J Biochem. 2009;146:463–469.
- Dogra V, Rochaix JD, Kim C. Singlet oxygen-triggered chloroplast-to-nucleus retrograde signalling pathways: An emerging perspective. Plant Cell Environ. 2018;41:1727–1738.
- Kato Y, Hyodo K, Sakamoto W. The Photosystem II repair cycle requires FtsH turnover through the EngA GTPase. Plant Physiol. 2018;178:596–611.
- Nishimura K, Kato Y, Sakamoto W. Chloroplast proteases: updates on proteolysis within and across suborganellar compartments. Plant Physiol. 2016;171:2280–2293.
- Zaltsman A, Ori N, Adam Z. Two types of FtsH protease subunits are required for chloroplast biogenesis and photosystem II repair in arabidopsis. Plant Cell. 2005;17:2782–2790.
- Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332:462–464.
- Kale R, Hebert AE, Frankel LK, Sallans L, Bricker TM, Pospisil P. Amino acid oxidation of the D1 and D2 proteins by oxygen radicals during photoinhibition of photosystem II. Proc Natl Acad Sci U S A. 2017;114:2988–2993.
- Dreaden Kasson TM, Rexroth S, Barry BA. Light-induced oxidative stress, n-formylkynurenine, and oxygenic photosynthesis. Plos One. 2012;7:e42220.
- Vainonen JP, Hansson M, Vener AV. STN8 protein kinase in arabidopsis thaliana is specific in phosphorylation of photosystem II core proteins. J Biol Chem. 2005;280:33679–33686.
