ABSTRACT
Polyamines (putrescine, spermidine and spermine) are ubiquitously present in various types of cells of living organisms. They are involved in a variety of cellular processes, including cell proliferation and cell differentiation, and are required for abiotic stress tolerances in plants. However, it is still not understood whether polyamines are involved in the plant growth inhibition caused by DNA-damaging agents. In this study, we examined the effects of polyamines on the inhibition of plant root growth and gene expression in Arabidopsis thaliana treated with mitomycin C (MMC), a genotoxic agent that induces DNA interstrand crosslinks. We found that polyamines alleviated the inhibitory effect caused by MMC on root growth. In addition, we also found that polyamines alleviated the increased expression of AtBRCA1 and AtRAD51 genes induced by MMC treatment. Our study provides the first evidence that polyamines contribute to tolerance against plant-growth inhibition caused by a DNA-damaging chemical.
Introduction
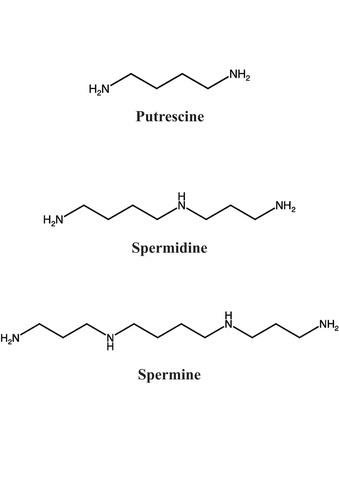
Polyamines are small polycationic molecules widely distributed in almost all living organisms.Citation1 Three major polyamines are often extremely abundant in eukaryotes: putrescine, spermidine and spermine (). Putrescine (a diamine) is converted to spermidine (a triamine) and then to spermine (a tetraamine) by sequential addition of aminopropyl groups donated by decarboxylated S-adenosylmethionine.Citation2 Numerous studies have shown that polyamines are involved in a variety of cellular processes, such as cell proliferation, cell differentiation, apoptosis and cellular senescence.Citation3,Citation4 Furthermore, it is well known that polyamines in plants increase a plant’s tolerance to abiotic stresses, including salinity, drought and chilling stress.Citation5,Citation6 In Arabidopsis thaliana (L.) Heynh., the lack of ACL5, a protein that catalyzes the biosynthesis of thermospermine (another tetraamine), strongly reduces stem elongation associated with an over-accumulation of lignin in vascular bundles.Citation7,Citation8 Thus, polyamines play important roles in various physiological processes involved in both reducing stress tolerance and in morphogenetic processes in plants.
Figure 1. Chemical structures of three polyamines, the diamine putrescine, the triamine spermidine and the tetraamine spermine.
Polyamines mainly bind to negatively charged macromolecules in cells, such as nucleic acids, phospholipids and particular acidic proteins.Citation9,Citation10 Polyamine/DNA interactions result in the duplex and triplex DNA stabilisations and induce DNA condensation.Citation11,Citation12 It has also been shown that polyamine depletion in HeLa cells is responsible for inhibiting DNA repair following X-ray-induced DNA strand breaks and production of UV light-induced pyrimidine dimers, Citation13,Citation14 suggesting that polyamines are involved in DNA-repair processes in cancer cells.
Mitomycin C (MMC), a DNA-damaging agent, interacts with DNA to induce interstrand crosslink (ICL), a covalent linkage formed between two DNA strands that are highly cytotoxic. ICL can be induced by several classes of carcinogens, such as MMC and cisplatin, and can be induced naturally by endogenous agents, such as formaldehyde and acetaldehyde.Citation15,Citation16 Williams et al. (1994) found that lung fibroblast V79 cells of Chinese hamsters in which polyamine is depleted by DFMO (an inhibitor of polyamine biosynthesis) are more sensitive to PUVA than control cells (without DFMO).Citation17 PUVA is an effective therapy for skin disorders because it induces ICL formation when patients are treated with psoralen in combination with UV-A irradiation therapy.Citation18 Therefore, polyamines likely provide a higher tolerance to cytotoxicity caused by ICL formation in cultured mammalian cells. However, it is still not understood how polyamines are involved in plant-growth inhibition caused by ICL-inducing agents. In this study, we focused on growth inhibition of primary roots caused by treating seedlings with MMC, examine the contribution of polyamines in counteracting the inhibition and investigate changes in gene expression in response to DNA damage.
Results and discussion
We first determined whether the growth of primary roots was affected by any of the polyamine treatments in the growth media. As shown in ), we detected no significant difference (P > .05) in primary root growths between roots in media containing 100 µM putrescine, spermidine or spermine and the control (without a polyamine). This suggests that these polyamines exert no discernible effect on the growth of primary roots, at least under the experimental conditions we imposed.
Figure 2. Extent to which polyamines alleviate the root-growth inhibition caused by MMC treatment. Panels: (a) the growth of primary roots after seedlings were transferred onto growth media with 100 µM of putrescine (Put), spermidine (Spd) or spermine (Spm) additions and the control condition (no polyamines), (b) the growth of primary roots after seedlings were transferred onto growth media with the different concentration of MMC (1, 3 and 5 µg/mL) and onto that without MMC, (c) the growth of primary roots after seedlings were transferred onto the media supplemented with 3 µg/mL MMC (MMC[+]) and polyamine hydrochloride (30 µM or 100 µM) media (Put, Spd or Spm) and a control medium without MMC [termed MMC[–]]. Error bars indicate standard deviation (n > 16). Significant differences between MMC[-] or polyamine-treated roots and their untreated roots were determined with the two-sample t-test at the P < .05 (*) level of significance. The n.s. represents no significant difference (P > .05).
![Figure 2. Extent to which polyamines alleviate the root-growth inhibition caused by MMC treatment. Panels: (a) the growth of primary roots after seedlings were transferred onto growth media with 100 µM of putrescine (Put), spermidine (Spd) or spermine (Spm) additions and the control condition (no polyamines), (b) the growth of primary roots after seedlings were transferred onto growth media with the different concentration of MMC (1, 3 and 5 µg/mL) and onto that without MMC, (c) the growth of primary roots after seedlings were transferred onto the media supplemented with 3 µg/mL MMC (MMC[+]) and polyamine hydrochloride (30 µM or 100 µM) media (Put, Spd or Spm) and a control medium without MMC [termed MMC[–]]. Error bars indicate standard deviation (n > 16). Significant differences between MMC[-] or polyamine-treated roots and their untreated roots were determined with the two-sample t-test at the P < .05 (*) level of significance. The n.s. represents no significant difference (P > .05).](/cms/asset/2d4852b3-8323-4936-ba56-3e023da8cd11/kpsb_a_1659687_f0002_oc.jpg)
Next, we tested the effect of different concentrations of MMC on the growth of primary roots. Our results showed that MMC inhibits the growth of primary roots depending on its concentration ()). Under our experimental conditions, it was often observed that the roots of seedlings cultivated on the 5 µg/mL MMC medium were grown to be exposed to air without touching on the agar medium, so we chose the concentration of 3 µg/mL MMC for further experiments to evaluate accurately the effect of MMC treatment.
To reveal the effect of polyamines on the growth of primary roots subjected to treatment with MMC, we compared the lengths of primary roots of seedlings grown on the medium containing MMC (termed MMC[+]) with added polyamines (putrescine, spermidine or spermine) and grown on a medium without any polyamines but with MMC[+]. ) shows that the polyamine supplements slightly alleviated the inhibition of growth associated with the addition of MMC to the growth media. For example, root lengths of seedlings treated with MMC[+] and 100 µM putrescine were 13.6% longer than without putrescine. Similar results were revealed for the two other polyamines: root lengths were 11.5% (30 µM spermidine) and 12.4% (100 µM spermidine) higher than controls, whereas they were 18.2% (30 µM spermine) and 26.9% (100 µM spermine) higher than the controls. Thus, all three polyamines we examined alleviated the inhibitory effect (due to MMC) on primary roots of A. thaliana seedlings.
To investigate whether the polyamines are involved in transcriptional regulation of DNA damage response (DDR) genes in A. thaliana, we examined the change of transcript abundance of the genes AtBRCA1 (At4g21070) and AtRAD51 (At5g20850). The BRCA1 is a human tumor suppressor that plays a critical role in DNA repair and cell-cycle checkpoints, whereas the RAD51 protein is a multifunctional protein implicated in DNA replication and homologous recombination repair.Citation19,Citation20 AtBRCA1 and AtRAD51 genes were isolated as A. thaliana orthologous genes of BRCA1 and RAD51.Citation21,Citation22 It has been previously reported that transcript levels of AtBRCA1 and AtRAD51 are markedly increased in A. thaliana by treating it with zeocin, a double strand break (DSB)-inducing agent.Citation23,Citation24
When we examined the MMC-dependent transcriptional changes of the AtBRCA1 and AtRAD51 genes with quantitative real-time PCR, AtBRCA1 and AtRAD51 gene expressions increased by approximately 8.8-fold and 7.2-fold, respectively, by 24 h after transferring seedlings to the MMC[+] medium ()). Next, we assessed the polyamine-dependent transcriptional changes of seedlings grown in the medium without MMC (termed MMC[–]) medium. Our results showed that putrescine, spermidine and spermine treatments were associated with lower expression of the AtBRCA1 gene than control (by 81.7%, 55.9% and 66.2% compared to the control on average, respectively), whereas expression of AtRAD51 declined by 71.4%, 56.0% and 70.9% as well (respectively). However, we found no significant difference (P > .05) in these gene expression with the addition of polyamines in the MMC[–] medium as determined by two-sample t-test.
Figure 3. Polyamine-dependent changes in AtBRCA1 and AtRAD51 gene expressions. Panels: (a) Relative gene expression level of AtBRCA1, (b) Relative gene expression level of AtRAD51. Expressions of AtBRCA1 and AtRAD51 were normalized using the ACT8 gene as an internal control. The values of control (Ctrl; no polyamine) with MMC[–] were set to 1.0 and other values were calculated relative to those. Error bars indicate standard deviation of biological triplicates. Significant differences between polyamine-treated seedlings and the untreated control for each series were determined with the two-sample t- test at P < .05 (*) level of significance.
![Figure 3. Polyamine-dependent changes in AtBRCA1 and AtRAD51 gene expressions. Panels: (a) Relative gene expression level of AtBRCA1, (b) Relative gene expression level of AtRAD51. Expressions of AtBRCA1 and AtRAD51 were normalized using the ACT8 gene as an internal control. The values of control (Ctrl; no polyamine) with MMC[–] were set to 1.0 and other values were calculated relative to those. Error bars indicate standard deviation of biological triplicates. Significant differences between polyamine-treated seedlings and the untreated control for each series were determined with the two-sample t- test at P < .05 (*) level of significance.](/cms/asset/8e45d944-82ab-48f5-9bff-a7d3ed25d61f/kpsb_a_1659687_f0003_oc.jpg)
We similarly assessed polyamine-dependent changes on the expression of AtBRCA1 and AtRAD51 genes of seedlings grown in MMC[+] medium. We found that treatment of all of three polyamines suppressed the increased expression of AtBRCA1 and AtRAD51 caused by MMC (,)). AtBRCA1 gene expression in the MMC[+] medium with putrescine, spermidine and spermine declined by 47.0%, 39.0% and 43.9% compared to the control (without polyamines), respectively, whereas AtRAD51 gene expression declined by 50.8%, 44.8% and 48.5% as well. These lower gene expression levels (relative to controls) were statistically significant (two-sample t-test, P < .05).
We showed that polyamines alleviated the inhibitory effect caused by MMC on the primary-root growth. Although Davis et al. (2016) already showed that several types of genotoxic agents (including MMC) inhibit the growth of primary roots and reduce lateral root density, Citation23 our study is the first to provide evidence that polyamines contribute to tolerance against plant growth inhibitions caused by a DNA-damaging chemical. In this study, we have no data to prove that the MMC treatments used in our experiments are enough to induce DNA cross-linking in the plant genome. However, it was shown that cross-linking was detected by a comet assay when the A. thaliana seedlings were treated with 3.3 µg/mL MMC after the application of an alkylating agent N-methyl-N-nitrosourea in a previous study.Citation25 Although our experimental procedures are not identical to the method in the precious study, there seems to be a possibility that the growth inhibition of primary roots induced by MMC treatment is due to a DNA cross-linking.
How are polyamines involved in the root-growth inhibition caused by MMC? It has been shown that polyamines interact genomic DNA to form nuclear aggregation.Citation26 Takata et al. (2013) reported that condensed chromatin of genomic DNA is better protected from DNA damages induced by DSB, heavy ions or genotoxic chemicals than when chromatin is not in a condensed condition.Citation27 One possibility is that polyamines may alleviate the inhibition of root growth (induced by MMC) by interacting with DNA to condense chromatin to prevent DNA from becoming damaged. Another possibility is that polyamines may play a role in facilitating DNA-repair activities, which in turn, consequently affects the expression of AtBRCA1 and AtRAD51 genes. A recent study showed that polyamines facilitate DSB repair by promoting homologous-recombination mediated by increased activity of the Rad51 protein.Citation28 Although MMC induces ICL, it also causes DNA strands to break by producing free radicals.Citation29 Further studies using DSB-inducing agents and reactive oxygen species in DNA-repair defective mutants are needed to determine whether molecular functions of polyamines on DNA damage is specific to a certain type of DNA damage or repair mechanism in plant cells.
Materials and methods
To evaluate changes in root growth following application of MMC and polyamines, the length of primary roots of wild-type seedlings of A. thaliana (Col-0 ecotype) were measured. First, seeds were sterilized with 70% ethanol for 5 min and then applied a sterilization solution [0.5% sodium hypochlorite, 0.05% Tween-20] for 10 min. After completing the sterilization process, the seeds were rinsed three times in sterile water and then placed on 0.8% agar medium [Murashige-Skoog (MS) medium with 2% sucrose (pH 5.8)]. Four-day-old seedlings were transferred to 1% agar MS medium with 2% sucrose (pH 5.8) containing various concentrations of MMC and polyamine salt (putrescine dihydrochloride, spermidine trihydrochloride or spermine tetrahydrochloride). Then the agar plates were set vertically in a growth chamber and subjected to continuous white light at 22°C. After the seedlings were grown for seven more days under these conditions, lengths of primary roots were measured.
For gene expression analysis, 2-week-old seedlings grown on the 0.8% MS agar plate were transferred to an MS liquid medium in a culture flask containing 100 µM polyamine-hydrochloride salt. The culture/seedling mixture was cultivated in the flask and by shaking it for 24 hours. Then, MMC was added to the culture flask to reach a final concentration of 3 µg/mL. After culturing the MMC/seedling mixture for 24 h, the seedlings were immediately frozen in liquid nitrogen and stored at −80 °C. Total RNAs were extracted from the frozen tissues using RNeasy Plant Mini Kit (QIAGEN) according to the manufacturer’s protocol, and reverse transcribed the RNA with iScriptTM Reverse Transcription Supermix (Bio-Rad) to conduct a quantitative reverse transcription polymerase chain reaction (PCR). Quantitative real-time PCR was performed on the CFX96 Real-Time PCR Detection System (Bio-Rad). The iQSYBR Green Supermix (Bio-Rad) intercalation dye was used as a fluorescent reporter and the following primers for the PCR analysis were used: BRCA1-F (5ʹ-AACCA CTGCA CTCTC AGCCT A-3ʹ), BRCA1-R (5ʹ-AGCCC TGAGC AAGAT AAGAC C-3ʹ), RAD51-F (5ʹ-CGCAA GTAGA TGGTT CAGCT C-3ʹ), RAD51-R (5ʹ-TCCTC TGCTC TTCCT TTCCT C-3ʹ), ACT8-F (5ʹ-CTCGT CGTCT TCAGC TTCAT C-3ʹ) and ACT-R (5ʹ-CGATG AAGAT CTGGC TCACT C-3ʹ). Relative expression levels for each sample were calculated on three biological replicates from standard curves. The expression levels of AtBRCA1 and AtRAD51 genes were normalized relative to the transcript levels of the actin gene ACT8 (At1g49240).
Declaration of interest statement
The authors declare no potential conflicts of interest.
Acknowledgments
The authors would like to thank Enago (www.enago.jp) for the English language review.
References
- Tabor CW, Tabor H. Polyamines. Annu Rev Biochem PubMed PMID: 6206782. 1984;53:749–790. doi:10.1146/annurev.bi.53.070184.003533.
- Heby O, Persson L. Molecular genetics of polyamine synthesis in eukaryotic cells. Trends Biochem Sci. 1990;15(4):153–158. PubMed PMID: 2187296.
- Cohen SS. A guide to the polyamines. New York, NY: Oxford University Press; 1998.
- Igarashi K, Kashiwagi K. Polyamines: mysterious modulators of cellular functions. Biochemical and Biophysical Research Communications. 2000;271(3):559–564. PubMed PMID: 10814501. doi:10.1006/bbrc.2000.2601.
- Groppa MD, Benavides MP. Polyamines and abiotic stress: recent advances. Amino Acids. 2008;34(1):35–45. PubMed PMID: 17356805. doi:10.1007/s00726-007-0501-8.
- Chen D, Shao Q, Yin L, Younis A, Zheng B. Polyamine function in plants: metabolism, regulation on development, and roles in abiotic stress responses. Front Plant Sci PubMed PMID: 30687350; PubMed Central PMCID: PMCPMC6335389. 2018;9:1945. doi:10.3389/fpls.2018.01945.
- Hanzawa Y, Takahashi T, Michael AJ, Burtin D, Long D, Pineiro M, Coupland G, Komeda Y. ACAULIS5, an Arabidopsis gene required for stem elongation, encodes a spermine synthase. The EMBO Journal. 2000;19(16):4248–4256. PubMed PMID: 10944107; PubMed Central PMCID: PMCPMC302034. doi:10.1093/emboj/19.16.4248.
- Kakehi J, Kuwashiro Y, Niitsu M, Takahashi T. Thermospermine is required for stem elongation in Arabidopsis thaliana. Plant Cell Physiol. 2008;49(9):1342–1349. PubMed PMID: 18669523. doi:10.1093/pcp/pcn109.
- Igarashi K, Kashiwagi K. Modulation of cellular function by polyamines. Int J Biochem Cell Biol. 2010;42(1):39–51. PubMed PMID: 19643201. doi:10.1016/j.biocel.2009.07.009.
- Schuster I, Bernhardt R. Interactions of natural polyamines with mammalian proteins. Biomol Concepts. 2011;2(1–2):79–94. PubMed PMID: 25962021. doi:10.1515/bmc.2011.007.
- Thomas T, Thomas TJ. Selectivity of polyamines in triplex DNA stabilization. Biochemistry. 1993;32(50):14068–14074. PubMed PMID: 8268186. doi:10.1021/bi00213a041.
- Saminathan M, Antony T, Shirahata A, Sigal LH, Thomas T, Thomas TJ. Ionic and structural specificity effects of natural and synthetic polyamines on the aggregation and resolubilization of single-, double-, and triple-stranded DNA. Biochemistry. 1999;38(12):3821–3830. PubMed PMID: 10090772. doi:10.1021/bi9825753.
- Snyder RD. Inhibition of X-ray-induced DNA strand break repair in polyamine-depleted HeLa cells. Int J Radiat Biol. 1989;55(5):773–782. PubMed PMID: 2565938.
- Snyder RD, Sunkara PS. Effect of polyamine depletion on DNA damage and repair following UV irradiation of HeLa cells. Photochem Photobiol. 1990;52(3):525–532. PubMed PMID: 2284346. doi:10.1111/j.1751-1097.1990.tb01795.x.
- Deans AJ, West SC. FANCM connects the genome instability disorders Bloom’s syndrome and Fanconi anemia. Mol Cell. 2009;36(6):943–953. PubMed PMID: 20064461. doi:10.1016/j.molcel.2009.12.006.
- Duxin JP, Walter JC. What is the DNA repair defect underlying Fanconi anemia? Curr Opin Cell Biol PubMed PMID: 26512453; PubMed Central PMCID: PMCPMC4688103. 2015;37:49–60. doi:10.1016/j.ceb.2015.09.002.
- Williams JR, Casero RA, Dillehay LE. The effect of polyamine depletion on the cytotoxic response to PUVA, gamma rays and UVC in V79 cells in vitro. Biochemical and Biophysical Research Communications. 1994;201(1):1–7. PubMed PMID: 8198560. doi:10.1006/bbrc.1994.1661.
- Momtaz K, Fitzpatrick TB. The benefits and risks of long-term PUVA photochemotherapy. Dermatol Clin. 1998;16(2):227–234. PubMed PMID: 9589196.
- Petermann E, Orta ML, Issaeva N, Schultz N, Helleday T. Hydroxyurea-stalled replication forks become progressively inactivated and require two different RAD51-mediated pathways for restart and repair. Mol Cell. 2010;37(4):492–502. PubMed PMID: 20188668; PubMed Central PMCID: PMCPMC2958316. doi:10.1016/j.molcel.2010.01.021.
- Rosen EM. BRCA1 in the DNA damage response and at telomeres. Front Genet PubMed PMID: 23802008; PubMed Central PMCID: PMCPMC3689208. 2013;4:85. doi:10.3389/fgene.2013.00085.
- Doutriaux MP, Couteau F, Bergounioux C, White C. Isolation and characterisation of the RAD51 and DMC1 homologs from Arabidopsis thaliana. Mol Gen Genet. 1998;257(3):283–291. PubMed PMID: 9520262. doi:10.1007/s004380050649.
- Lafarge S, Montane MH. Characterization of Arabidopsis thaliana ortholog of the human breast cancer susceptibility gene 1: AtBRCA1, strongly induced by gamma rays. Nucleic Acids Research. 2003;31(4):1148–1155. PubMed PMID: 12582233; PubMed Central PMCID: PMCPMC150221. doi:10.1093/nar/gkg202.
- Davis OM, Ogita N, Inagaki S, Takahashi N, Umeda M. DNA damage inhibits lateral root formation by up-regulating cytokinin biosynthesis genes in Arabidopsis thaliana. Genes Cells. 2016;21(11):1195–1208. PubMed PMID: 27658920. doi:10.1111/gtc.12436.
- Huang CH, Mirabelli CK, Jan Y, Crooke ST. Single-strand and double-strand deoxyribonucleic acid breaks produced by several bleomycin analogues. Biochemistry. 1981;20(2):233–238. PubMed PMID: 6162480. doi:10.1021/bi00505a001.
- Menke M, Chen I, Angelis KJ, Schubert I. DNA damage and repair in Arabidopsis thaliana as measured by the comet assay after treatment with different classes of genotoxins. Mutat Res. 2001;493(1–2):87–93. PubMed PMID: 11516718. doi:10.1016/s1383-5718(01)00165-6.
- Iacomino G, Picariello G, D’Agostino L. DNA and nuclear aggregates of polyamines. Biochimica et biophysica acta. 2012;1823(10):1745–1755. PubMed PMID: 22705882. doi:10.1016/j.bbamcr.2012.05.033.
- Takata H, Hanafusa T, Mori T, Shimura M, Iida Y, Ishikawa K, Yoshikawa K, Yoshikawa Y, Maeshima K. Chromatin compaction protects genomic DNA from radiation damage. PLoS One. 2013;8(10):e75622. PubMed PMID: 24130727; PubMed Central PMCID: PMCPMC3794047. doi:10.1371/journal.pone.0075622.
- Lee CY, Su GC, Huang WY, Ko MY, Yeh HY, Chang GD, Lin SJ, Chi P. Promotion of homology-directed DNA repair by polyamines. Nature Communications. 2019;10(1):65. PubMed PMID: 30622262; PubMed Central PMCID: PMCPMC6325121. doi:10.1038/s41467-018-08011-1.
- Doroshow JH. Role of hydrogen peroxide and hydroxyl radical formation in the killing of Ehrlich tumor cells by anticancer quinones. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(12):4514–4518. PubMed PMID: 3086887; PubMed Central PMCID: PMCPMC323764. doi:10.1073/pnas.83.12.4514.
