ABSTRACT
The galling insect manipulates the host plant tissue to its own benefit, building the gall structure where it spends during most of its life cycle. These specialist herbivore insects can induce and manipulate plant structure and metabolism throughout gall development and may affect plant volatile emission. Consequently, volatile emission from altered metabolism contribute to eavesdropping cueing. Eavesdropping can be part of adaptive strategies used by evolution for both galling insects and the entire-associated community in order to cue some interaction response. This is in contrast to some herbivores associated with delayed induced responses, altering plant metabolites during the short time while they feed. Due to the different lifestyles of the galling organism, which are associated with different plant tissues and organs (e.g leaves, flowers or fruits), a distinct diversity of organisms may eavesdrop on induced volatiles interacting with the galls. Furthermore, the eavesdropping cues may be defined according to the phenological coupling between galling organism and host plant, which results from the development of a gall structure. For instance, when plants release volatile-induced defenses after galling insects’ activity, another interactor may perceive these volatiles and change its behavior and interactions with host plants and galls. Thus, natural enemies could be attracted by different volatiles emitted by the gall tissues. Considering the duration of the life cycle of the galling organism and the gall, the temporal extent of gall-induced volatiles may include more persistent volatile cues and eavesdropping effects than the volatiles induced by non-galling herbivores. Accordingly, from chemical ecology perspective we expect that galling herbivore-induced volatiles may exhibit robust effects on neighboring-plant interactions including those ones during different plant developmental or phenological periods. Information about multitrophic interactions between insects and plants supports the additional understanding of direct and indirect effects, and allows insight into new hypotheses.
Introduction
Plants produce a blend of organic compounds, including the volatiles (VOCs), which may directly and indirectly affect other plants, herbivores, and their natural enemies, pollinators and seed dispersers.Citation1–Citation3 Plant volatiles induce local, systemic, and interspecific responses, even in the absence of vascular connection, assuming self or eavesdrop cueing.Citation4 Volatile cues are part of remarkable strategies of plants for growth and development, but environmental factors such as climate change and biodiversity changes may activate and modulate the activity of plant signaling at the molecular level.Citation5,Citation6 Different organisms, especially plants, perceive and respond to peripheral volatiles by adjusting traits to improve reproductive performance; thus, eavesdropping or overhearing on plant volatile cues may be an adaptive strategy.Citation4,Citation7 Thus, plant volatiles may be used as cues by neighbors, including other plants (competitors) herbivores, their natural enemies, as well the mutualistic partners (), according to their traits and conditions, and thus provide information about insect-plant and/or plant–plant interactions.Citation9,Citation10
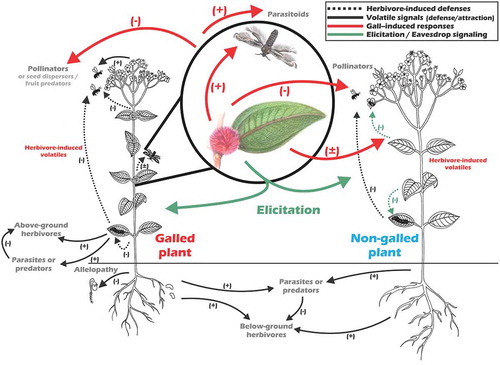
Figure 1. Hypothetical framework of plant–plant interactions by volatile organic compoundsCitation8 which, due to different gall life stages, could temporally permit events ranging from pollinator attraction to allopathy in order to avoid competition (black arrows). The plant volatiles are induced as defensive mechanisms in response to herbivores and consequently affect herbivore activity (dashed black arrows), directly by repelling them or indirectly by attracting herbivore enemies (e.g. parasitoids). However, assuming that herbivory induced responses on plants (wider and red arrows) are temporally extended when compared with other herbivores. According to this, in addition to the direct and indirect effects this temporal extention of responses may include effect both above and below-ground organisms and interactions related to the infested plants and their neighboring community. These gall-induced responses may affect neighboring plants by eliciting or allowing eavesdropping on olfactory cues (green arrows). Among them, elicitation from gall metabolism may enable plants, including the non-galled ones, to exhibit specific herbivore-induced responses. Particularly, it allows to hypothesize that gall cues signals to neighboring plants can be either positive – reducing the attractiveness of gallers or attracting natural enemies of galls – or negative – by indicating to the galling insects possible host plants. These signals may be used for both, pollinators, seed dispersers and parasitoids also to avoid or fing resources on plants infested by galling insects directing them to specific niches. For instance, volatile cues from induced defense may induce parasitoids to plants with galling insects presence, and other herbivores or pollinators may use thes cues to avoid galled plants with possibly lower resources available.
Herbivores modify plant metabolism according to their feeding habit (e.g. sapping or chewing) and active responsive and specific mechanisms related to herbivore traits, such as different life stage feeding and salivary compounds or herbivore-associated molecular patterns (HAMPs) exposed during herbivore damage.Citation11,Citation12 Induced volatile emission in response to herbivory attack could function as direct and indirect defense by being understood as cue that induces herbivores to finish their attack or it can promote effective defense release by vegetal tissues.Citation9,Citation13 Volatiles released from feeding herbivores could act as an indirect plant defense since these molecules may also attract natural enemies of herbivores and thus benefit the plant.Citation14 However, some herbivores such as caterpillars have evolved using plant volatile cues for their own benefit, increasing the attractiveness of conspecific caterpillars to the damaged plant.Citation15,Citation16,Citation17 The blend composition of volatiles may be modified after herbivore damage, acting as herbivore-induced defenses and interfering with interactions between other organisms.Citation10,Citation18 Additionally, a constant induced defense may include trade-off resolutions on plant fitness, which may reproduce or defense less than previous periods according to the cues recognizing between neighboring plants.Citation19
Herbivory effects: plant trait modifications induced throughout herbivore development
Galls represent an interesting structure developing from the host plant tissue after induction mainly by insects and resulting in changes in host plant metabolism.Citation20,Citation21 Using unknown mechanical and chemical stimuli, the galling insects induce galls with different tissues from those of the host plant. These tissues are a gain for the galling insect in relation to its free-life ancestors, giving better nutrition and defense.Citation22,Citation23,Citation24 Thus, the galling insects manipulate the host plant morphogenesis and metabolism for their own benefit, while completing their development using host plants resources.Citation25,Citation26,Citation27
The intimate relationship between galling insects and host plant organs can lead to gall formation with different and specialized tissues such as lignified and nutritive tissues.Citation23 The nutritive tissue can be induced by different taxa of galling organisms and is responsible for their nutrition.Citation28 Primary compounds (e.g. carbohydrates, proteins and lipids) are commonly detected in this nutritive tissue by galling insects and used in their diet.Citation29 However, secondary compounds (e.g. phenolics derivatives) are distributed in the outer cortices of galls, commonly associated with mechanical tissue.Citation30,Citation31 These secondary compounds in the outer cortices of galls can provide defenses against natural enemies.Citation32,Citation33 As a plant organ induced by insects,Citation21 galls can accumulate large amounts of defensive compounds,Citation34 with herbivore induction being responsible for most of the defensive responses.Citation12,Citation35 Within this context, the constitutive and induced plant defense strategies may be changed by herbivores, including the gall system interactions, which activate elicitors from the myriad signaling pathways related to direct and indirect plant defenses.Citation36
All herbivores alter plant metabolism during feeding activity, but some herbivores such as galling insects are more likely to induce or manipulate plant tissues during an extended period of feeding during their development.Citation27,Citation37 However, galling activity depends on the chemical and mechanical reactive host plant tissues, as well as on synchronization between the life cycle of the galling organism and host plant conditions (e.g. phenology).Citation38,Citation39 The galling stimuli for the manipulation and development of the gall seem to be constant over its life cycle.Citation40 The optimal defense theories predict less costs from inducible defenses than from constitutive ones because those are produced only when they are actually needed.Citation41 However, the time lag after biotic or abiotic cue and the optimal adjust of plant defensive mechanisms still is considered a strong disadvantage of inducible defenses.Citation42,Citation43 Thus, considering that time as an important factor in plant defense strategies,Citation44 such volatile emissions, we believe that galling-plant interactions may constantly influence other insect-plant interactions – including mutualist and antagonistic ones – in natural communities ().
Histochemical and physiological assays have helped to elucidate the chemical interactions between the dietary requirements of the galls and the chemical defensive arsenal of the host plants.Citation31 nonvolatile plant chemistry is known to be altered in the gall and surrounding leaf tissue.Citation45 Thus, the chemical changes in plant traits due to gall activity are associated with the feeding and protection strategies of galling insects in order to complete their developmental stages.Citation39,Citation46 Some of these strategies are complex and induce chemical trait changes in plant tissues including nectar availability from galls, which support the protection of galling insects.Citation47 The development of galling insects depends on the nutritional status, and particularly on the physiology and phenology of their host plantsCitation48 as they recognize the reactive tissue.Citation38 An adequate nutritional status, as well as synchronized galling insects’ life cycles and plant phenology, are required for gall initiation and development,Citation49 in addition to strategies used to explore the host plant resources leading to gall phenotype.Citation39 The gall phenotype may depend on the length of the life cycle of the galling insects. Insects with short life cycles appear to develop less structurally and metabolically complex galls than galling insects with long life cycles, regardless of inductor taxon, as shown by their structural and physiological profiles.Citation25,Citation48 Thus, the longer the galling insect lives in the gall, the greater its chance of interacting with the community of animals around.
Galls are able to modify herbivore-induced responses against other herbivores due to changes in plant tissue,Citation37,Citation50 but the VOC emission from gall-infested plants can vary greatly among species of galling insects on the same plant.Citation51 In Baccharis dracunculifolia, galls are induced by the psyllid Baccharopelma dracunculifoliae to produce 3.5% more VOCs than usual;Citation52 Pistacia atlantica galled by the aphid Slavum wertheimae produced approximately 2.5 times more volatiles than ungalled plants; and galls induced by the aphids Baizongia pistaciae on Pistacia palaestina exhibited independent metabolism producing and accumulating monoterpenes.Citation33 These examples emphasize the ability of galling insects to alter the biochemical function of the host plant, possibly protecting them against their own natural enemies.Citation53 Due to plant immobility, the volatiles induced by galling insects may be an efficient strategy to avoid galls’ natural enemies or plant’s herbivores, specifically considering the possible manipulation of VOC release.Citation54 Changes in volatiles induced by galls can increase or decrease the amount of VOCs emitted (), but evidence suggests that gall formation mainly affects the emission of terpenoids and VOCs from the lipoxygenase pathway, common volatile cues involved in direct and indirect defenses.Citation50,Citation51 Therefore, VOCs essentially mediate diverse ecological interactions but the understanding of VOCs induced by galling activity remains unclear, especially considering long-term effects in addition to interactions of other insects with the host plant and plant phenology. To better explore the host plant resources the galling insect must synchronize their colonization activities with the host plant phenology, in an essential step of the interaction establishment. This fine-tuned synchronization and the continuous galling stimuli can lead to univoltine life cycle along one year time,Citation39 thus extending the long term of effects VOCs induced by the galls.
The ecological role of gall-emitted volatiles as cues for surrounding insect-plant interactions
The chewers and phloem-sucking herbivores may induce not only immediate, but also delayed defense responses, and alter plant metabolites during feeding or on an extended time after feeding.Citation19,Citation43,Citation55 However, galling herbivores may induce and manipulate plant tissues throughout their developmental processes and may also induce delayed responses. Released VOCs are temporarily able to react with other molecules or organismsCitation5,Citation56 and their lifetime varies (minutes to hours) depending on environmental conditions such as air pollution.Citation57 However, galling insects affect plant traits and maintain modifications for a longer time than other non-galling insect herbivores.
In a general way, the development of galls depends on the continuous chemical, physical and feeding stimuli of the galling organismCitation28,Citation58 and how much the plant tissues are reactiveness to the galling organisms.Citation38,Citation39 As an example, Bystracoccus mataybae (Eriococcidae) can induce gall in both stems and leaflets of Matayba guianensis (Sapindaceae). These galls induced by the same galling organism showed different morphological and anatomical traits, a clear indicative of constraints imposed by the different organ of the host plant.Citation59 Despite this, the histochemical profile detected in the leaflet gall tissues is established in the first steps of gall development, which is maintained until gall maturation by the feeding action of the galling insect.Citation60 Another example horn-shaped gall induced by Cecidomyiidae on leaflets of Copaifera langsdorffii (Fabaceae) has an univoltine life cycle along one-year time. This galling insect continuously stimulates the host tissue and consequently, the gall showed a complex structural and histochemical profile.Citation25 Therefore, considering galling herbivores as constant inducers during their developmental processes, new approaches comparing effects between galling and non-galling herbivores could expect the effects of galls on plant metabolism to be constantly inducing or avoiding plant defenses, in contrast to non-galling herbivores.
Complementarily, the idea that surrounding changes in the environment may affect insect-plant interactions is well accepted, especially regarding plant volatile cueing which may be disrupted due the noise from other possible environmental interactive volatiles.Citation61,Citation62 Considering the herbivore-induced volatile effects on other organisms which interact with the surrounding community, galling insects are expected to affect the dynamics of their host plants () and a large set of interactions including other herbivores,Citation63 pollinatorsCitation17 or neighboring plants.Citation7 For instance, based on the morphological variations selected by plant-galling interactions, different sizes, colors, and fresh floral fragrances can be incorporated into populations affecting their community-level interactions, such as plant-pollinator-herbivore interactions.Citation10 Changes in floral characters related to the attraction of pollinators can affect the floral visitors’ behavior and the frequency of their visits and may culminate in the pollinator exchange.Citation64 Changes in flower coloration have been poorly studied within the biology of pollination and, even less frequently, within the context of interaction with galling insects. However, the composition or concentration of VOCs may also be altered by the action of herbivores on vegetative or reproductive vegetative tissue.Citation3
Gall induction and development depends not only on specific host traits,Citation27,Citation48 but also on galling recognition by their natural enemies and other competing herbivores possibly through the eavesdropping.Citation37,Citation65 Since different organisms recognizes the galls, several positive or negative effects may be encompassed on host plants interactions (). Additionally, the galling insects may use a wide range of volatile cues released by plants to avoid those that pinpoint non-ideal periods for gall induction.Citation66 It has also been observed that some monoterpenes serve as olfactory cues for host location by the gall wasp Antistrophus rufus, helping the female to find optimal host plant species and to choose better ovipositional ones.Citation67 The gall wasp Antistrophus rufus induces host plant volatiles which attract male wasps and enhance their mating with partners.Citation68 In this system, the intraspecific competition between male gall-forming insects and females could be reduced by two mechanisms: (I) galls recognizing and locating host plants by volatile compounds also released from reactive and healthy plant tissue – including healthy plants on this scenery – and (II) galling activity suppressing the normal release of plant volatiles.Citation12,Citation37 Focusing on intraspecific galling insects interactions, it is expected that changes in volatiles release – both from the induced healthy plants and those suppressed by gallers – may inactivate olfactory cues for intraspecific galling insects and consequently reduce the male competition for females ().
Similarly, when we transpose this idea to interactions involving predators (or parasitoids), the predatory pressure is reduced when there is less volatile emission acting as olfactory cues for prey location. For instance, Solidago altissima exhibited enhanced defenses and reduced susceptibility to insect feeding damage when previously exposed to volatiles from the galling insect Eurosta solidaginis.Citation69 It is not clear whether plant reaction to gall induction is related to herbivore defenses or if the modifications of plant metabolism actively guide the galls (e.g. avoid defensive effects).Citation37,Citation65 In mango leaves, there is an enhancement of VOCs in gall fly-susceptible cultivars compared to resistant ones, clearly relating VOC emission to gall fly susceptibility.Citation70 Moreover, Silphium laciniatum galled by Antistrophus rufus increases the plant volatile production employed as an olfactory cue by the parasitoid females (Euritoma lutea).Citation71 Consequently, this gall-parasitoid interaction helps plant fitness by reducing galling insect infestation.Citation5,Citation72
Salicylate accumulation by galls may act on volatile suppression, as well as on resource limitation and salivary components, which consequently make herbivores less affected by plant defense.Citation37 Thus, plant defense suppression by herbivores is widely unexplored and its particular effects may lead us to believe that galling suppressors act as ecosystem engineers. The beetle Microrhopala vittata prefers to colonize Solidago altissima plants that were galled by Rhopalomyia solidaginis, suggesting a possible advantage of aggregating on galled plants.Citation50 On P. atlantica, the galling aphid Slavum wertheimae, which enhances release of leaf volatile terpenes, also reduces plant palatability and consequently reduces herbivory of galled tissue.Citation33 Both of these studies revealed a significant impact of galls on host plant quality by suggesting through metabolic changes that insect preference and interacting behavior is plant mediated by gall facilitation effects on plant palatability.Citation33,Citation50 Thus, the different developmental gall stages and plant genotypes show that the gall–plant interaction is a synergistic process.Citation73,Citation74 Additionally, the phenological association between plant and galling organism life cycles represents a new frontier of chemical ecology, which determines how the VOCs released during the host plant phenological stages interact with the galling insects, natural enemies or host plant interactors such as pollinators.
Accordingly, the herbivore-induced volatiles vary due to different stimuli, and concomitantly depend on herbivore habit and damage, allowing wide possible interaction responses. From an ecological point of view, plant community may mediate effects from galling herbivores to all possible organisms they interact. Therefore, in order to highlight our perspective about galling herbivore-induced defenses may affect ecological interactions differently from non-galling ones, we emphasize that galling insects may induce volatiles during all processes of gall development – not only during feeding – and consequently affect a large set of interactions since roots on soil until flowers on plant top (). Nevertheless, it refers to question such: how are host plants chemically restructured by galling organisms? And, how do they provide cues for neighboring organisms and their respective interactions?
Answering these questions could include understanding on plant defenses induced by galling herbivores, and its effects on nearby ecological interactions. To better understand the ecological role of volatile cues from galls is necessary (i) to compare volatile emission between galling and non-galling herbivores, (ii) to record the induced volatile emission from galled tissue during different times along gallers development, and (iii) to measure if and how the neighboring interactions are affected along time while gall develops and induces defenses on plants. Therefore, we strongly suggest that new research efforts could be focused on understanding how volatile cues mediate ecological interactions in real-world environments and on the ecological and evolutionary implications of chemical cues from phenotypes within and among plant populations. Although the questions still need to be answered, mainly regarding the suppressive mechanisms associated with trait-mediated effects, the knowledge about multitrophic interactions between insects and plants supports the understanding of direct and indirect effects and allows insight into new hypotheses.
Declaration of interest statement
The authors declare there is no any financial interest that arisen from direct application of this paper.
Acknowledgments
We would like to thank Moshe Inbar from University of Haifa – Israel for criticism and considerations during manuscript preparation. GJB is grateful to PELD/CAPES/CNPq/UFU for postdoctoral fellowships (nº 88887.137914/2017-00) and CAPES/PNPD/UFVJM (nº 88887.352134/2019-00) and to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for funding (Universal nº 425130/2018-5). DCO is grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for a produtivity fellowship (nº 301246/2016-5). All authors are grateful for color illustration support provided by Ana Carolina Monetta.
Additional information
Funding
References
- Benelli G, Canale A, Romano D, Flamini G, Tavarini S, Martini A, Ascrizzi R, Conte G, Mele M, Angelini LG. Flower scent bouquet variation and bee pollinator visits in Stevia rebaudiana Bertoni (Asteraceae), a source of natural sweeteners. Arthropod Plant Interact. 2017;11:1–7. doi:10.1007/s11829-016-9488-y.
- Karban R, Yang LH, Edwards KF. Volatile communication between plants that affects herbivory: a meta-analysis. Ecol Lett. 2014;17:44–52. doi:10.1111/ele.12205.
- Lucas-Barbosa D, Sun P, Hakman A, van Beek TA, van Loon JJA, Dicke M. Visual and odour cues: plant responses to pollination and herbivory affect the behaviour of flower visitors. Funct Ecol. 2016;30:431–441. doi:10.1111/1365-2435.12509.
- Caruso CM, Parachnowitsch AL. Do plants eavesdrop on floral scent signals? Trends Plant Sci. 2016;21:9–15. doi:10.1016/j.tplants.2015.09.001.
- Heil M. Herbivore-induced plant volatiles: targets, perception and unanswered questions. New Phytol. 2014;204:297–306. doi:10.1111/nph.12977.
- Blande JD, Glinwood R. Deciphering chemical language of plant communication. Cham. 2016. doi:10.1007/978-3-319-33498-1.
- Ninkovic V, Markovic D, Dahlin I. Decoding neighbour volatiles in preparation for future competition and implications for tritrophic interactions. Perspect Plant Ecol Evol Syst. 2016;23:11–17. doi:10.1016/j.ppees.2016.09.005.
- Dudareva N, Negre F, Nagegowda DA, Orlova I. Plant volatiles: recent advances and future perspectives. CRC Crit Rev Plant Sci. 2006;25:417–440. doi:10.1080/07352680600899973.
- Lucas-Barbosa D, Van Loon JJA, Dicke M. The effects of herbivore-induced plant volatiles on interactions between plants and flower-visiting insects. Phytochemistry. 2011;72:1647–1654. doi:10.1016/j.phytochem.2011.03.013.
- Kessler A, Halitschke R. Testing the potential for conflicting selection on floral chemical traits by pollinators and herbivores: predictions and case study. Funct Ecol. 2009;23:901–912. doi:10.1111/j.1365-2435.2009.01639.x.
- Howe GA, Jander G. Plant immunity to insect herbivores. Annu Rev Plant Biol. 2008;59:41–66. doi:10.1146/annurev.arplant.59.032607.092825.
- Kant MR, Jonckheere W, Knegt B, Lemos F, Liu J, Schimmel BCJ, Villarroel CA, Ataide LMS, Dermauw W, Glas JJ, et al. Mechanisms and ecological consequences of plant defence induction and suppression in herbivore communities. Ann Bot. 2015;115:1015–1051. doi:10.1093/aob/mcv054.
- De Boer JG, Hordijk CA, Posthumus MA, Dicke M. Prey and non-prey arthropods sharing a host plant: effects on induced volatile emission and predator attraction. J Chem Ecol. 2008;34:281–290. doi:10.1007/s10886-007-9405-z.
- Baldwin IT, Kessler A, Halitschke R. Volatile signaling in plant–plant–herbivore interactions: what is real? Curr Opin Plant Biol. 2002;5:351–354. doi:10.1016/S1369-5266(02)00263-7.
- El-Sayed AM, Knight AL, Byers JA, Judd GJR, Suckling DM. Caterpillar-induced plant volatiles attract conspecific adults in nature. Sci Rep. 2016;6:37555. doi:10.1038/srep37555.
- Turlings TCJ, Erb M. Tritrophic interactions mediated by herbivore-induced plant volatiles: mechanisms, ecological relevance, and application potential. Annu Rev Entomol. 2018;63:433–452. doi:10.1146/annurev-ento-020117-043507.
- Fatouros NE, Lucas-Barbosa D, Weldegergis BT, Pashalidou FG, van Loon JJA, Dicke M, Harvey JA, Gols R, Huigens ME. Plant volatiles induced by herbivore egg deposition affect insects of different trophic levels. PLoS One. 2012;7:e43607. doi:10.1371/journal.pone.0043607.
- Cozzolino S, Fineschi S, Litto M, Scopece G, Trunschke J, Schiestl FP. Herbivory increases fruit set in Silene latifolia: A consequence of induced pollinator-attracting floral volatiles? J Chem Ecol. 2015;41:622–630. doi:10.1007/s10886-015-0597-3.
- Benevenuto RF, Hegland SJ, Töpper JP, Rydgren K, Moe SR, Rodriguez-Saona C, Seldal T. Multiannual effects of induced plant defenses: are defended plants good or bad neighbors? Ecol Evol. 2018;8:8940–8950. doi:10.1002/ece3.4365.
- Raman A. Insect-induced plant galls of India: unresolved questions. Curr Sci. 2007;92:748–757.
- Shorthouse JD, Wool D, Raman A. Gall-inducing insects – nature’s most sophisticated herbivores. Basic Appl Ecol. 2005;6:407–411. doi:10.1016/j.baae.2005.07.001.
- Rozdilsky ID, Stone L, Solow A. The effects of interaction compartments on stability for competitive systems. J Theor Biol. 2004;227:277–282. doi:10.1016/j.jtbi.2003.11.007.
- Stone GN, Schönrogge K. The adaptive significance of insect gall morphology. Trends Ecol Evol. 2003;18:512–522. doi:10.1016/S0169-5347(03)00247-7.
- Price PW, Fernandes GW, Waring GL. Hypotheses on the adaptive nature of galls. Proc Entomol Soc Washingt. 1986;16:15–24.
- Carneiro RGS, Isaias RMS, Moreira ASFP, Oliveira DC. Reacquisition of new meristematic sites determines the development of a new organ, the cecidomyiidae gall on Copaifera langsdorffii Desf. (Fabaceae). Front Plant Sci. 2017:8. doi:10.3389/fpls.2017.01622.
- Ferreira BG, Isaias RMDS. Floral-like destiny induced by a galling Cecidomyiidae on the axillary buds of Marcetia taxifolia (Melastomataceae). Flora - Morphol Distrib Funct Ecol Plants. 2014;209:391–400. doi:10.1016/j.flora.2014.06.004.
- Raman A. Morphogenesis of insect-induced plant galls: facts and questions. Flora - Morphol Distrib Funct Ecol Plants. 2011;206:517–533. doi:10.1016/j.flora.2010.08.004.
- Bronner R. The role of nutritive cells in the nutrition of cynipids and cecidomyiids. In: Shorthouse J, Rohfrtisch O editors. Biology of insect-induced galls. Oxford: Oxford University Press; 1992. p. 118–140.
- Ferreira BG, Avritzer SC, Isaias RMS. Totipotent nutritive cells and indeterminate growth in galls of Ditylenchus gallaeformans (Nematoda) on reproductive apices of Miconia. Flora. 2017;227:36–45. doi:10.1016/j.flora.2016.12.008.
- Nyman T, Julkunen-Tiitto R. Manipulation of the phenolic chemistry of willows by gall-inducing sawflies. Proc Natl Acad Sci. 2000;97:13184–13187. doi:10.1073/pnas.230294097.
- Kuster VC, Costa Rezende U, Fernandes Cardoso JC, Dos Santos Isaias RM, Coelho de Oliveira D. How galling organisms manipulate the secondary metabolites in the host plant tissues?: A histochemical overview in neotropical gall systems. In: Merillon J, Ramawat K, editors. Co-evolution of secondary metabolites. 2019; p. 1–20. doi:10.1007/978-3-319-76887-8_29-1.
- Allison SD, Schultz JC. Biochemical responses of chestnut oak to a galling cynipid. J Chem Ecol. 2005;31:151–166.
- Rostás M, Maag D, Ikegami M, Inbar M. Gall volatiles defend aphids against a browsing mammal. BMC Evol Biol. 2013;13:193. doi:10.1186/1471-2148-13-193.
- Chehab EW, Kaspi R, Savchenko T, Rowe H, Negre-Zakharov F, Kliebenstein D, Dehesh K. Distinct roles of jasmonates and aldehydes in plant-defense responses. PLoS One. 2008;3:1–10. doi:10.1371/journal.pone.0001904.
- Kanchiswamya CN, Maffeib ME. Calcium signaling preceding the emission of plant volatiles in plant-insect interactions. J Indian Inst Sci. 2015;95:15–23.
- Aljbory Z, Chen M-S. Indirect plant defense against insect herbivores: a review. Insect Sci. 2018;25:2–23. doi:10.1111/1744-7917.12436.
- Tooker JF, Rohr JR, Abrahamson WG, De Moraes CM. Gall insects can avoid and alter indirect plant defenses. New Phytol. 2008;178:657–671. doi:10.1111/j.1469-8137.2008.02392.x.
- Weis AE, Walton R, Crego CL. Reactive plant tissue sites and the population biology of gall makers. Annu Rev Entomol. 1988;33:467–486. doi:10.1146/annurev.en.33.010188.002343.
- Oliveira DC, Isaias RMS, Fernandes GW, Ferreira BG, Carneiro RGS, Fuzaro L. Manipulation of host plant cells and tissues by gall-inducing insects and adaptive strategies used by different feeding guilds. J Insect Physiol. 2016;84:103–113. doi:10.1016/j.jinsphys.2015.11.012.
- Speed MP, Fenton A, Jones MG, Ruxton GD, Brockhurst MA. Coevolution can explain defensive secondary metabolite diversity in plants. New Phytol. 2015;208:1251–1263. doi:10.1111/nph.13560.
- Karban R, Agrawal AA, Thaler JS, Adler LS. Induced plant responses and information content about risk of herbivory. Trends Ecol Evol. 1999;14:443–447. doi:10.1016/S0169-5347(99)01678-X.
- Padilla DK, Adolph SC. Plastic inducible morphologies are not always adaptive: the importance of time delays in a stochastic environment. Evol Ecol. 1996;10:105–117. doi:10.1007/BF01239351.
- Backmann P, Grimm V, Jetschke G, Lin Y, Vos M, Baldwin IT, van Dam NM. Delayed chemical defense: timely expulsion of herbivores can reduce competition with neighboring plants. Am Nat. 2019;193:125–139. doi:10.1086/700577.
- Niinemets Ü, Kännaste A, Copolovici L. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Front Plant Sci. 2013;4:1–15. doi:10.3389/fpls.2013.00262.
- Albert S, Padhiar A, Gandhi D, Nityanand P. Morphological, anatomical and biochemical studies on the foliar galls of Alstonia scholaris (Apocynaceae). Rev Bras Botânica. 2011;34:343–358. doi:10.1590/S0100-84042011000300009.
- Nabity PD, Haus MJ, Berenbaum MR, DeLucia EH. Leaf-galling phylloxera on grapes reprograms host metabolism and morphology. Proc Natl Acad Sci. 2013;110:16663–16668. doi:10.1073/pnas.1220219110.
- Aranda-Rickert A, Rothen C, Diez P, González AM, Marazzi B. Sugary secretions of wasp galls: a want-to-be extrafloral nectar? Ann Bot. 2017;120:765–774. doi:10.1093/aob/mcx075.
- Oliveira DC, Mendonça MS, Moreira ASFP, Lemos-Filho JP, Isaias RMS. Water stress and phenological synchronism between Copaifera langsdorffii (Fabaceae) and multiple galling insects: formation of seasonal patterns. J Plant Interact. 2013;8:225–233. doi:10.1080/17429145.2012.705339.
- Dorchin N, Hoffman JH, Stirk WA, Novãk O, Strnad M, Van Staden J. Sexually dimorphic gall structures correspond to differential phytohormone contents in male and female wasp larvae. Physiol Entomol. 2009;34:359–369. doi:10.1111/j.1365-3032.2009.00702.x.
- Uesugi A, Morrell K, Poelman EH, Raaijmakers CE, Kessler A. Modification of plant-induced responses by an insect ecosystem engineer influences the colonization behaviour of subsequent shelter-users. J Ecol. 2016;104:1096–1105. doi:10.1111/1365-2745.12587.
- Jiang Y, Veromann-Jürgenson -L-L, Ye J, Niinemets Ü. Oak gall wasp infections of Quercus robur leaves lead to profound modifications in foliage photosynthetic and volatile emission characteristics. Plant Cell Environ. 2018;41:160–175. doi:10.1111/pce.13050.
- Besten MA, Nunes DS, Granato D, Sens SL, Wisniewski A Jr, Simionatto EL, Riva-Scharf D. Volatile components from galls induced by Baccharopelma dracunculifoliae (Hemiptera: psyllidae) on leaves of Baccharis dracunculifolia (Asteraceae). Quim Nova. 2014. doi:10.5935/0100-4042.20140288.
- Rand K, Bar E, Ari MB, Davidovich-Rikanati R, Dudareva N, Inbar M, Lewinsohn E. Differences in monoterpene biosynthesis and accumulation in Pistacia palaestina leaves and aphid-induced galls. J Chem Ecol. 2017;43:143–152. doi:10.1007/s10886-016-0817-5.
- Stireman JO, Cipollini D. Stealth tactics of galling parasites and their potential indirect effects. New Phytol. 2008;178:462–465. doi:10.1111/j.1469-8137.2008.02455.x.
- Frost CJ, Mescher MC, Carlson JE, De Moraes CM. Plant defense priming against herbivores: getting ready for a different battle. Plant Physiol. 2008;146:818–824. doi:10.1104/pp.107.113027.
- Kessler A. Defensive function of herbivore-induced plant volatile emissions in nature. Science (80-). 2001;291:2141–2144. doi:10.1126/science.291.5511.2141.
- Holopainen JK, Blande JD. Where do herbivore-induced plant volatiles go? Front Plant Sci. 2013;4:1–13. doi:10.3389/fpls.2013.00185.
- Mani MS. Ecology of Plant Galls. Dordrecht: Springer Netherlands; 1964. doi:10.1007/978-94-017-6230-4.
- Pfeffer L, Rezende UC, Barônio GJ, de Oliveira DC. Building two houses on a singles host plant: galling insect synchronizes its life cycle with plant phenology. Oecologia Aust. 2018;22:438–448. doi:10.4257/oeco.2018.2204.07.
- Silva AFDM, Kuster VC, Rezende UC, de Oliveira DC. The early developmental stages of gall-inducing insects define final gall structural and histochemical profiles: the case of Bystracoccus mataybae galls on Matayba guianensis. Botany. 2019;97:427–438. doi:10.1139/cjb-2019-0017.
- Burkle LA, Runyon JB. The smell of environmental change: using floral scent to explain shifts in pollinator attraction. Appl Plant Sci. 2017;5:1600123. doi:10.3732/apps.1600123.
- Li T, Blande JD, Holopainen JK. Atmospheric transformation of plant volatiles disrupts host plant finding. Sci Rep. 2016;6:33851. doi:10.1038/srep33851.
- Borges RM. The galling truth: limited knowledge of gall-associated volatiles in multitrophic interactions. Front Plant Sci. 2018:9. doi:10.3389/fpls.2018.01139.
- Giron D, Huguet E, Stone GN, Body M. Insect-induced effects on plants and possible effectors used by galling and leaf-mining insects to manipulate their host-plant. J Insect Physiol. 2016;84:70–89. doi:10.1016/j.jinsphys.2015.12.009.
- Tooker JF, De Moraes CM. Gall insects and indirect plant defenses: A case of active manipulation? Plant Signal Behav. 2008;3:503–504. doi:10.4161/psb.3.7.6184.
- Helms AM, De Moraes CM, Mescher MC, Tooker JF. The volatile emission of Eurosta solidaginis primes herbivore-induced volatile production in Solidago altissima and does not directly deter insect feeding. BMC Plant Biol. 2014;14:173. doi:10.1186/1471-2229-14-173.
- Tooker JF, Crumrin AL, Hanks LM. Plant volatiles are behavioral cues for adult females of the gall wasp Antistrophus rufus. Chemoecology. 2005;15:85–88. doi:10.1007/s00049-005-0298-4.
- Tooker JF, Koenig WA, Hanks LM. Altered host plant volatiles are proxies for sex pheromones in the gall wasp Antistrophus rufus. Proc Natl Acad Sci. 2002;99:15486–15491. doi:10.1073/pnas.252626799.
- Helms AM, De Moraes CM, Tooker JF, Mescher MC. Exposure of Solidago altissima plants to volatile emissions of an insect antagonist (Eurosta solidaginis) deters subsequent herbivory. Proc Natl Acad Sci. 2013;110:199–204. doi:10.1073/pnas.1218606110.
- Augustyn WA, Botha BM, Combrinck S, Maree JE, Du Plooy GW. Effect of secondary metabolites on gall fly infestation of mango leaves. Flavour Fragr J. 2010;25:223–229. doi:10.1002/ffj.1999.
- Tooker JF, Hanks LM. Tritrophic interactions and reproductive fitness of the prairie perennial Silphium laciniatum Gillette (Asteraceae). Environ Entomol. 2006;35:537–545. doi:10.1603/0046-225X-35.2.537.
- Dicke M, Baldwin IT. The evolutionary context for herbivore-induced plant volatiles: beyond the “cry for help”. Trends Plant Sci. 2010;15:167–175. doi:10.1016/j.tplants.2009.12.002.
- Li XQ, Liu YZ, Guo WF, Solanki MK, Yang ZD, Xiang Y, Ma ZC, Wen YG. The gall wasp Leptocybe invasa (Hymenoptera: eulophidae) stimulates different chemical and phytohormone responses in two Eucalyptus varieties that vary in susceptibility to galling. Tree Physiol. 2017;37:1208–1217. doi:10.1093/treephys/tpx098.
- Kotze MJ, Jürgens A, Johnson SD, Hoffmann JH. Volatiles associated with different flower stages and leaves of Acacia cyclops and their potential role as host attractants for Dasineura dielsi (Diptera: cecidomyiidae). South African J Bot. 2010;76:701–709. doi:10.1016/j.sajb.2010.07.024.
