ABSTRACT
Late embryogenesis abundant (LEA) proteins are highly hydrophilic and thermostable proteins that could be induced by abiotic stresses in plants. Previously, we have isolated a group 3 LEA gene WZY3-1 (GenBank: KX090360.1) in wheat. In this study, the recombinant plasmid with WZY3-1 was transformed into Escherichia coli BL21 for protein expression. Furthermore, we transformed WZY3-1 into Arabidopsis. Overexpression of WZY3-1 in E.coli enhanced their tolerance to mannitol and NaCl. WZY3-1 protein could protect lactate dehydrogenase (LDH) under freeze and heat stress. Overexpression of WZY3-1 showed that WZY3-1 could help to improve the drought tolerance of transgenic Arabidopsis. In summary, our works show that WZY3-1 plays an important role in abiotic stress resistance in prokaryotic and eukaryotic organisms.
Introduction
Late embryogenesis abundant (LEA) proteins, originally found in cotton seeds, belong to a large group protein family that maintain the water content in plant cells under abiotic stresses.Citation1 During abiotic stresses (drought, cold, high salinity, etc.), the accumulation of LEA proteins increased dramatically in various organs of the plant and some LEA proteins accumulation position varied after stresses.Citation2 An Arabidopsis LEA protein ERD14 mainly expressed in leaf, root and stem under normal condition. However, under cold stress, the accumulation of ERD14 was detected throughout the entire plant.Citation3 Most of LEA proteins are hydrophilic and thermostable. They possess high ratios of charged amino acids such as Lys and Gly and lack of uncharged amino acid like Cys and Tyr. Although the protective mechanisms of LEA proteins are unclear, many studies have indicated that LEA protein can inhibit aggregation of proteins/enzymes, and bind to excessive metal ions and lipids under low temperature and desiccationCitation4–Citation8
LEA proteins can be classified into seven protein subfamilies, among which the group 3 of LEA protein are identified by an 11-mer amino acid motif (TAQAAKEKAGE)Citation9 and the conserved 11-mer amino acids play an important role in LEA 3 proteins acquiring α-helical conformations during dehydration.Citation10-Citation13 Both in silico predictions and spectroscopic experiments demonstrate that LEA 3 proteins are mainly intrinsically disordered proteins (IDPs). Some study on LEA 3 proteins also revealed that a typical IDP AcLEA could protect E.coli against desiccation, oxidant, and osmotic stresses through clear conformational variation detected by circular dichroism (CD), Fourier transform infrared spectrometer (FTIR) and nuclear magnetic resonance (NMR) (Saucedo et al., 2017). The model peptide of group 3 LEA protein G3LEA from the larvae of P. vanderplanki is involved in the structural transition from random coils to α-helix in drought stress.Citation10 Study on TdLEA3 showed that drying, trifluoroethanol (TFE) and glycerol were capable of inducing α-helical structure from disordered state of TdLEA3. Moreover, TdLEA3 was able to prevent the inactivation of lactate dehydrogenase (LDH) under heat, dehydration-rehydration and freeze-thaw treatments via reducing the aggregate formation of LDH.Citation14 ZmLEA3 could participate in preventing aggregation of α-casein, LDH via disordered-to-order conformations in vitro, in addition, ZmLEA3 could improve low-temperature tolerance in transgenic yeast, E.coli, and overexpression plants.Citation6 Overexpression of a wheat LEA gene in Phellodendron amurense indicated that TaLEA3 could improve the drought resistance by inducing rapid stomatal clousure in transgnic lines, and further experiments indicating that TaLEA3 overexpression could positively regulate nitric oxide (NO) biosynthesis and accumulation in the guard cells.Citation15 Similar to TaLEA3, a LEA gene named AtLEA3 from Arabidopsis also enhanced the drought tolerance in overexpression lines, and seed size, membrane stability and seed weight were also improved in overexpression lines.Citation16
Although previous works have revealed part functions of LEA 3 proteins, the roles of LEA 3 in plant abiotic stress tolerance are still ambiguous. In this work, overexpression of WZY3-1 in E.coli and Arabidopsis resulted in improved tolerance to drought and salt stress.
Materials and methods
Expression of WZY3-1 in E.coli
The cDNA of WZY3-1 was kept in our lab. WZY3-1 was inserted into the PET28a vector and then transformed into E.coil BL21(DE3) in order to obtain the recombinant proteins of WZY3-1. The recombinant protein with His-tag at N-terminus was purified through Ni2+ Heparin-Sepharose affinity chromatography and then identified by Western blot. His antibody was selected for the Western bolt.
LDH protection assay
10 μg/mL LDH (Sigma, USA) from rabbit muscle was mixed with 10 mM sodium phosphate buffer (pH 7.4) and WZY3-1 protein. In the low-temperature assay, the mixture was frozen for 1 min in liquid nitrogen and thawed for 10 min in a water bath at 25°C. In the high-temperature assay, the mixture was heated for 30 min in a water bath at 45°C. The reaction mixture contained 10 mM pyruvic acid and 2 mM nicotinamide adenine dinucleotide (NADH). LDH activity was measured as the rate of decrease in absorbance at 340 nm for 5 min using a microplate reader (SpectraMax M2, Molecular Devices, USA) due to the oxidation of NADH. The rate determined for the untreated samples was considered as 100% in our experiments.
Abiotic stresses tolerance assays of E.coli transformants
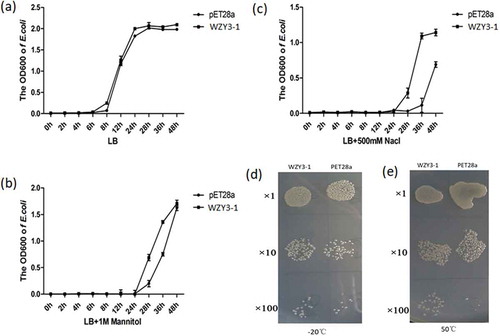
The recombinant E.coil was cultured in lysogeny broth (LB) medium containing 0.5 mM isopropyl β-D-thiogalactoside (IPTG) for 6 h, then 400 μL E.coil solution was added to 15 ml LB contains 500 mM NaCl and 1 M mannitol, respectively. At each time point, 3 ml of culture was used to measure the absorbance at 600 nm. For temperature treatment, the culture was cooled in −20°C for 2h or heated in 50°C for 1 h.
Plant materials and growth condition
The Columbia Arabidopsis was used in this study. The Arabidopsis seeds were grown in a commercial soil mix under 200 μE m−2s−1 light at 20/23°C (night/day) temperature with a 16-h photoperiod.
Arabidopsis transformation and abiotic stress tolerance analysis
WZY3-1 was cloned into PBI121 and driven by CaMV 35S promoter. The construct was then transferred into Agrobacterium GV3101. The Arabidopsis plants were transformed by the floral dip methodCitation17 and homozygous T3 seeds were used.
For osmotic stress assay, wild-type and transgenic Arabidopsis were cultivated into 1/2 Murashige and Skoog (MS) medium for 10 d, then transferred them into the new 1/2 MS vertical medium that contained 0 mM, 150 mM, 200 mM, 250 mM, 300 mM, 400 mM mannitol, respectively. For drought stress treatment, Arabidopsis ecotype Columbia plants and transgenic Arabidopsis were cultivated in the soil for 4 weeks and then stop to the water supply.
Measurements of malondialdehyde (MDA), proline, chlorophyll and water content
Rosette leaves of Arabidopsis were gathered to measure the physiological parameters. MDA contents and proline contents were evaluated as described previously.Citation18 Chlorophyll contents were measured as described previously.Citation19 For water content assay, the samples were dried at 98°C to 100°C until the constant dry weight was achieved, generally expressed on a percentage fresh weight basis.
Quantitative RT-PCR analysis
In order to detect the expression pattern of WZY3-1, CPK3, CPK6, GHR1, HT1, OST, and SLAC1, total RNA was isolated from Arabidopsis with or without treatment (trizol). cDNA was synthesized from RNA using TransScript One-Step gDNA Removal and cDNA Synthesis Supermix (TransGen Biotech, Beijing) according to its manual. QRT-PCR was implemented using BIO-RAD CFX96 and TransStart Tip Green qPCR SuperMix (TransGen Biotech, Beijing). Each sample was measured three biological replicates.
Stomatal aperture
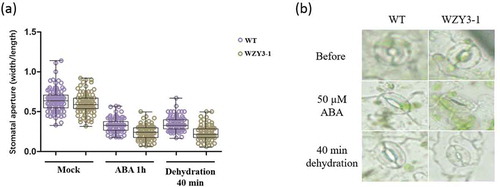
Three leaves of wild-type and WZY3-1 transgenic Arabidopsis were cut and the lower surface was immediately stuck to a microspore slide with a medical adhesive. After 1 min, the leaf was peeled away under distilled water. The lower epidermis stuck to the glass was visualized under the microscope and stomatal images were recorded. Measurements of stomatal aperture were performed using the imaging software Image J, version 6.
Statistical analysis
All of the experiments in this study were repeated three times in independent experiments, and the data shown are the mean ± SD. In this research, statistical analyzes were performed using the statistical tools (Student’s t-test) of Microsoft Excel software.
Results
Purification and acquisition of WZY3-1 protein
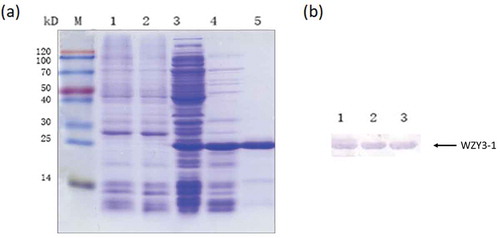
Prokaryotic expression method was used in our assay to obtain the WZY3-1 protein. The protein was purified rely on His-tag of its terminal (). To further validate the recombinant protein is WZY3-1, we carried out Western blot assay and the results demonstrate that we obtained the WZY3-1 protein and the molecular weight confirmed to our projection ().
Figure 1. Analysis of induced expression products by SDS-PAGE (a) and Western Blot (b).
(a) M: Protein Marker; 1: The supernatant obtained after heat from E. coli cultures producing pET28a with IPTG; 2: The supernatant obtained after heat from E. coli cultures producing pET28a without IPTG; 3: The supernatant obtained after cell disruption from E. coli cultures producing WZY3-1; 4: The supernatant obtained after cell disruption and heat; 5: WZY3-1 protein purified with Ni2+ agarose gel affinity column. (b) M: Protein Marker; 1–3: protein purified with Ni2+ agarose gel affinity column.
WZY3-1 protected ldh activity under freezing and thermal stress conditions
Since LDH loses its activity during freezing and thermal conditions and a number of LEA proteins can protect LDH protein. We selected this enzyme to evaluate the protective effect of WZY3-1. LDH activity before treatment was regarded as 100%. After freezing treatment, the activity of LDH was 97% in the presence of WZY3-1. In contrast, the activity of LDH was only 27% in sodium phosphate buffer. For thermal treatment, similarly, the activity of LDH was 26% in sodium phosphate buffer but in the presence of WZY3-1, the activity of LDH higher than 80% ().
Figure 2. Protection of WZY3-1 protein on LDH activity under freezing and heat treatment. The of LDH activities were measured under freezing (f1, f2, f3) and heat (ht1, ht2, ht3) treatment with three cycles. Asterisks indicate statistically significant differences (*P < .05, **P < .01; Student’s t-test).
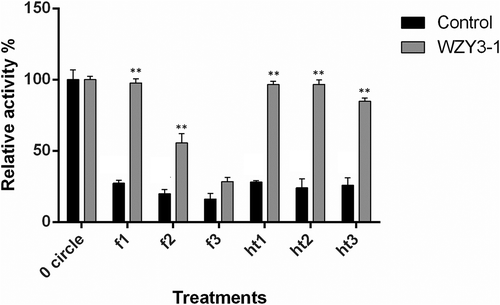
In summary, obviously, WZY3-1 can protect the activity of proteins from damage caused by freezing and thermal stresses.
Overexpression of WZY3-1 enhanced the viability of E.coli under abiotic stresses conditions
To explore whether WZY3-1 protein could enhance abiotic stress tolerance in vivo, the growth of the recombinant E.coil was examined to represent the function of WZY3-1 in abiotic stress. Under optimal conditions, there was no obvious growth difference the control and transformed lines. However, in the presence of 400 mM NaCl or 400 mM mannitol, the growth condition of the transformed line was superior to the control (–). Similarly, in freezing or thermal stresses, the transformed line showed higher viability than the control after culture for 24h (,).
Overexpression of WZY3-1 enhanced transgenic arabidopsis tolerance to abiotic stresses
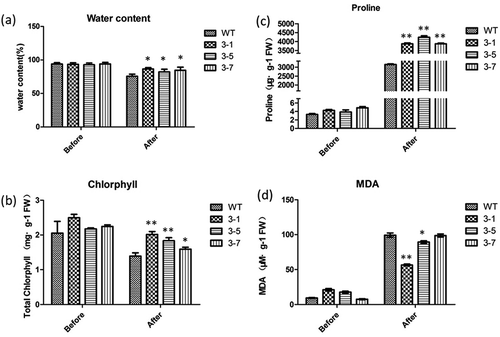
To further elucidate the function of the WZY3-1 protein in plant tolerance to abiotic stresses, independent transgenic lines were obtained by Kanamycin-resistance selection and confirmed by genomic PCR. The semi-quantitive RT-PCR assay was used to detect the expression levels of WZY3-1 in Arabidopsis (). Three independent WZY3-1 transgenic lines (T3-1, T3-5, T3-7), with higher transgenic expressions, were detected for their tolerance under abiotic stresses. In mannitol treatment assay, when the concentration of mannitol exceed 150 mM, the root length of the transgenic plants longer than WT plants until the concentration of mannitol more than 300 mM (,). After drought stress treatment, the transgenic lines exhibited greener leaves than WT plants (). These results indicate that overexpression of WZY3-1 increases plants tolerance to osmotic and drought stress. To further confirm the tolerance of transgenic lines in drought stress, physiological and biochemical indexes of transgenic plants was measured, the results show that the water content and chlorophyll content of transgenic plants was higher than WT plant after drought treatment, it is noteworthy that the proline content of transgenic lines showed a sharp rise. On the contrary, the MDA contents of transgenic lines were lower than WT plants ().
Figure 4. The screening of transgenic Arabidopsis. (a) The semi-quantitative test of wildtype (WT) and transgenic Arabisopsis (line 3–1, 3–5 and 3–7). (b) The growth state of WT and transgenic Arabisopsis under mannitol treatment. (c) The root length of WT and transgenic Arabidopsis under mannitol treatment. (d) The growth state of WT and transgenic Arabidopsis under drought stress for 0, 6, 7, 8 and 10 days. Asterisks indicate statistically significant differences (*P < .05, **P < .01; Student’s t-test).
Figure 5. Measurement of physiological and biochemical indexes of transgenic and wild type plants treated with drought. (a) The water content of WT and transgenic Arabidopsis. (b) The total chlorphyll content of WT and transgenic Arabidopsis. (c) The proline content of WT and transgenic Arabidopsis. (d) The MDA content of WT and transgenic Arabidopsis. Asterisks indicate statistically significant differences (*P < .05, **P < .01; Student’s t-test).
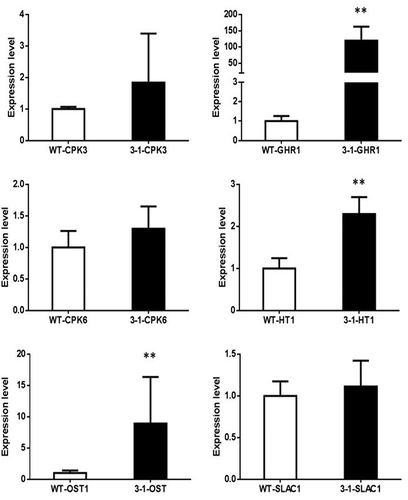
In addition, we also measured the stomatal aperture of WT and transgenic Arabidopsis under normal and ABA/Dehydration treatment (). The results showed a decreased aperture of stomata in both WT and transgenic plants under 1h ABA and 40 min dehydration treatment. However, the stomata apertures in transgenic plants are lower than WT (Mock) lines. To further elucidate the relationship between WZY3-1 and stomata closure, we implemented qPCR of seven stomatal movement-related genes, including calcium-dependent protein kinase 3 (CPK3), calcium-dependent protein kinase 6 (CPK6), Guard Cell Hydrogen Peroxide-resistance 1(GHR1), HIGH LEAF TEMPERATURE1 (HT1), OPEN STOMATA (OST1) and SLOW ANION CHANNEL1 (SLAC1) (). The results of qPCR demonstrated the expression of GHR1, HT1 and OST1 were significantly up-regulated in transgenic plants under drought conditions. Taken together, these results demonstrated that WZY3-1 was involved in plant tolerance to abiotic stresses.
Discussion
At present, a series of studies have uncovered that LEA3 proteins could protect the plant from environmental stresses by their unique protein structure and intrinsically disordered property.Citation4,Citation6,Citation7,Citation20,Citation21 LEA proteins are highly hydrophilic, and are speculated to protect other proteins/enzymes from aggregation or desiccation.Citation22 LDH as a reporter enzyme have been widely used to test the protection activity of different group LEA proteins, and it is hypothesized that this protection activity is implemented via chaperone molecules of LEA protein.Citation14,Citation22,Citation23 In our study, the result showed that WZY3-1 could protect the LDH activity from damage caused by freezing and heat cycles (). Previous study indicated that LEA protein could function via formation of dimers that forming a large molecular shield around biomolecules. The result indicated WZY3-1 may protect LDH activity by stabilizing enzyme under environmental stress.
Overexpression of LEA genes in many plants has successfully indicated their enhancement of abiotic stress tolerance.Citation6,Citation7 Overexpression of a barley LEA gene HVA1 in transgenic rice improved the tolerance to water deficit and salt stress.Citation24 Further study also showed overexpression of HVA1 could enhance the growth of rice root.Citation25 Recent analysis of transgenic study confirmed that LEA3 could alter lipid accumulation via enhancing photosynthetic efficiency and reducing ROS content.Citation16
In this study, the transgenic Arabidopsis showed longer root length under osmotic stress, indicating that WZY3-1 could enhance transgenic Arabidopsis tolerance to osmotic stress. Furthermore, the transgenic Arabidopsis also exhibited higher water content, proline total chlorophyll, and lower MDA content. According to those results, we could speculate that WZY3-1 could enhance transgenic Arabidopsis tolerance to drought stress.
Group 3 LEA proteins are not only widely distributed in the eukaryotic organisms, but also existed in prokaryotes such as Deinovoccus radioduransCitation26 and Acidovorax avenae.Citation27 MeLEA3 from Manihot esculenta is charaterised to confer a protective function against heat and salt stress in bacterial cells.Citation28 To investigate whether WZY3-1 could protect prokaryotic organisms, E.coli BL21 was chosen for further study. The expression of WZY3-1 in E.coli significantly increased its tolerance to osmotic, salt, heat and cold stresses. The results implying that some common protective mechanisms in both prokaryotes and eukaryotes under abiotic stress. In addition, we also observed stomatal closure of Arabidopsis under ABA treatment. Our results showed the stomatal apertures of transgenic Arabidopsis were significantly reduced to wild-type.
Stomatal aperture is largely regulated by cell turgor, which is controlled by solutes and water in guard cells. The stomatal closure is caused by efflux of anion through anion channels in guard cell plasma membrane, the plasma membrane are then depolarized and thr outward cation channels are activated, which results in efflux of solutes and decreased turgor in guard cells.Citation29 The protein kinase HT1 has a central role in CO2-induced staomatal regulation.Citation30 Other than HT1, several guard cell regulators have also been characterized. The disruption of SLAC1 expression could impaired the stomatal closure induced by ozone, CO2, ABA and calcium, which implied that the SLAC protein family is essential for the plasma membrane of S-type anion channel function in guard cells.Citation31,Citation32 SLAC1 could be phosphorylated by OST1, which is a serine/threonine protein kinase that positively regulate ABA-mediated stomatal responses.Citation33 Smilar to OST1, a gene named GHR1, which encodes a receptor-like kinase localized on the plasma membrane in Arabidopsis, could also interact and activate S-type anion channel SLOW ANION CHANNEL-ASSOCIATED1.Citation34 Further study also uncovered that SLAC1 HOMOLOG3 (SLAH3) also contributes to S-type anion channel and essential for nitrate transport in the roots.Citation35,Citation36 Recent study discovered that SLAC1/SLAH3 participated in signaling pathways for stomatal closure and opening in Arabidpopsis.Citation37 CPKs are widely distributed in the plant, and CPKs appear to play a dual role in abiotic stress response. Genetic study have uncovered that AtCPK3 and AtCPK6 mediate ABA responses in guard cells, and they participate in calcium activation in slow-type anion channels.Citation38 In addition, AtCPK3 is found to contribute to salt stress by phosphorylating the vacuolar two-pore K+ channel (TPK1).Citation39 In our study, the qRT-PCR of stomatal movement related genes also implied that overexpression of WZY3-1 could up-regulate GHR1, HT1 and OST1 (), which showed a positive role of LEA proteins in enhancing the closure of stomata under abiotic conditions and ABA treatment, which is coherent to CaLEA1 and TaLEA3.Citation15,Citation40 However, the WZY3-1 could regulate the stomatal movement without the significant expression of CPK3, CPK6 and SLAC1, indicating a complex regulation of LEA gene and ABA-mediated stomatal movement that is essential to explore in futher study.
In summary, our works show that WZY3-1 plays an important role in abiotic stress resistance in E.coli and Arabidopsis, but the molecular mechanism to elucidate how WZY3-1 participated in abiotic stress responses are still ambiguous, thus, further experiments are necessary to explore this question.
Authors’ contributions
LZ and ZY designed the study, wrote part of the manuscript, and finalized the figures. XW and YT carried out most of the experiments and data analysis and wrote part of the manuscript. ZY, XW, YT and DZ performed experiments and took care of plants. All authors have read and approved the manuscript.
Disclosure of potential conflicts of interest
The authors declare no conflict of interest.
Additional information
Funding
References
- Dure L 3rd, Crouch M, Harada J, Ho TH, Mundy J, Quatrano R, Thomas T, Sung ZR. Common amino acid sequence domains among the LEA proteins of higher plants. Plant Mol Biol. 1989;12:1–8. doi:10.1007/BF00036962.
- Houde M, Daniel C, Lachapelle M, Allard F, Laliberte S, Sarhan F. Immunolocalization of freezing-tolerance-associated proteins in the cytoplasm and nucleoplasm of wheat crown tissues. Plant J. 1995;8:583–593. doi:10.1046/j.1365-313x.1995.8040583.x.
- Nylander M, Svensson J, Palva ET, Welin BV. Stress-induced accumulation and tissue-specific localization of dehydrins in Arabidopsis thaliana. Plant Mol Biol. 2001;45:263–279. doi:10.1023/a:1006469128280.
- Furuki T, Sakurai M. Group 3 LEA protein model peptides protect enzymes against desiccation stress. Biochim Biophys Acta. 2016;1864:1237–1243. doi:10.1016/j.bbapap.2016.04.012.
- Liu Y, Wang L, Jiang S, Pan J, Cai G, Li D. Group 5 LEA protein, ZmLEA5C, enhance tolerance to osmotic and low temperature stresses in transgenic tobacco and yeast. Plant Physiol Biochem. 2014;84:22–31. doi:10.1016/j.plaphy.2014.08.016.
- Liu Y, Liang J, Sun L, Yang X, Li D. Group 3 LEA Protein, ZmLEA3, Is Involved in Protection from Low Temperature Stress. Front Plant Sci. 2016;7:1011.
- Liu Y, Wang L, Xing X, Sun L, Pan J, Kong X, Zhang M, Li D. ZmLEA3, a multifunctional group 3 LEA protein from maize (Zea mays L.), is involved in biotic and abiotic stresses. Plant Cell Physiol. 2013;54:944–959. doi:10.1093/pcp/pct047.
- Furuki T, Shimizu T, Chakrabortee S, Yamakawa K, Hatanaka R, Takahashi T, Kikawada T, Okuda T, Mihara H, Tunnacliffe A, et al. Effects of Group 3 LEA protein model peptides on desiccation-induced protein aggregation. Biochim Biophys Acta. 2012;1824:891–897. doi:10.1016/j.bbapap.2012.04.013.
- Dure L 3rd. A repeating 11-mer amino acid motif and plant desiccation. Plant J. 1993;3:363–369. doi:10.1046/j.1365-313x.1993.t01-19-00999.x.
- Furuki T, Shimizu T, Kikawada T, Okuda T, Sakurai M. Salt Effects on the structural and thermodynamic properties of a group 3 LEA protein model peptide. Biochemistry. 2011;50:7093–7103. doi:10.1021/bi200719s.
- Hatanaka R, Hagiwara-Komoda Y, Furuki T, Kanamori Y, Fujita M, Cornette R, Sakurai M, Okuda T, Kikawada T. An abundant lea protein in the anhydrobiotic midge, pvlea4, acts as a molecular shield by limiting growth of aggregating protein particles. Insect Biochem Mol Biol. 2013;43:1055–1067.
- Liu Y, Yang M, Cheng H, Sun N, Liu S, Li S, Wang Y, Zheng Y, Uversky VN. The effect of phosphorylation on the salt-tolerance-related functions of the soybean protein pm18, a member of the group-3 lea protein family. Biochim Biophys Acta Proteins Proteom. 2017;1865:1291–1303.
- Saucedo AL, Hernandez-Dominguez EE, de Luna-Valdez LA, Guevara-Garcia AA, Escobedo-Moratilla A, Bojorquez-Velazquez E, Del Rio-Portilla F, Fernandez-Velasco DA, Barba De La Rosa AP. Insights on Structure and Function Of a Late Embryogenesis Abundant Protein from Amaranthus Cruentus: an Intrinsically Disordered Protein Involved in Protection against Desiccation, Oxidant Conditions, and Osmotic Stress. Front Plant Sci. 2017;8:497.
- Koubaa S, Bremer A, Hincha DK, Brini F. Structural properties and enzyme stabilization function of the intrinsically disordered LEA_4 protein TdLEA3 from wheat. Sci Rep. 2019;9:3720. doi:10.1038/s41598-019-39823-w.
- Yang J, Zhao S, Zhao B, Li C. Overexpression of TaLEA3 induces rapid stomatal closure under drought stress in Phellodendron amurense Rupr. Plant Sci. 2018;277:100–109. doi:10.1016/j.plantsci.2018.09.022.
- Liang Y, Kang K, Gan L, Ning S, Xiong J, Song S, Xi L, Lai S, Yin Y, Gu J, et al. Drought-responsive genes, late embryogenesis abundant group3 (LEA3) and vicinal oxygen chelate, function in lipid accumulation in Brassica napus and Arabidopsis mainly via enhancing photosynthetic efficiency and reducing ROS. Plant Biotechnol J. 2019. doi:10.1111/pbi.13127.
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi:10.1046/j.1365-313x.1998.00343.x.
- Zhang L, Zhao G, Xia C, Jia J, Liu X, Kong X. A wheat R2R3-MYB gene, TaMYB30-B, improves drought stress tolerance in transgenic Arabidopsis. J Exp Bot. 2012;63:5873–5885.
- Pflugmacher S, Hofmann J, Hubner B. Effects on growth and physiological parameters in wheat (Triticum aestivum L.) grown in soil and irrigated with cyanobacterial toxin contaminated water. Environ Toxicol Chem. 2007;26:2710–2716. doi:10.1897/07-145.1.
- Furuki T, Sakurai M. Group 3 LEA protein model peptides protect liposomes during desiccation. Biochim Biophys Acta. 2014;1838:2757–2766. doi:10.1016/j.bbamem.2014.07.009.
- Boswell LC, Hand SC. Intracellular localization of group 3 LEA proteins in embryos of Artemia franciscana. Tissue Cell. 2014;46:514–519. doi:10.1016/j.tice.2014.09.004.
- Tunnacliffe A, Wise MJ. The continuing conundrum of the LEA proteins. Naturwissenschaften. 2007;94:791–812. doi:10.1007/s00114-007-0254-y.
- Kovacs D, Agoston B, Tompa P. Disordered plant LEA proteins as molecular chaperones. Plant Signal Behav. 2008;3:710–713. doi:10.4161/psb.3.9.6434.
- Xu D, Duan X, Wang B, Hong B, Ho T, Wu R. Expression of a Late Embryogenesis Abundant Protein Gene, HVA1, from Barley Confers Tolerance to Water Deficit and Salt Stress in Transgenic Rice. Plant Physiol. 1996;110:249–257. doi:10.1104/pp.110.1.249.
- Chen YS, Lo SF, Sun PK, Lu CA, Ho TH, Yu SM. A late embryogenesis abundant protein HVA1 regulated by an inducible promoter enhances root growth and abiotic stress tolerance in rice without yield penalty. Plant Biotechnol J. 2015;13:105–116. doi:10.1111/pbi.12241.
- Battista JR, Park MJ, McLemore AE. Inactivation of two homologues of proteins presumed to be involved in the desiccation tolerance of plants sensitizes Deinococcus radiodurans R1 to desiccation. Cryobiology. 2001;43:133–139. doi:10.1006/cryo.2001.2357.
- Goyal K, Tisi L, Basran A, Browne J, Burnell A, Zurdo J, Tunnacliffe A. Transition from natively unfolded to folded state induced by desiccation in an anhydrobiotic nematode protein. J Biol Chem. 2003;278:12977–12984. doi:10.1074/jbc.M212007200.
- Barros NL, Da Silva DT, Marques DN, de Brito FM, Dos Reis SP, de Souza CR. Heterologous Expression of MeLEA3: A 10 kDa Late Embryogenesis Abundant Protein of Cassava, Confers Tolerance to Abiotic Stress in Escherichia coli with Recombinant Protein Showing In Vitro Chaperone Activity. Protein Pept Lett. 2015;22:689–695.
- Kim TH, Bohmer M, Hu H, Nishimura N, Schroeder JI. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol. 2010;61:561–591. doi:10.1146/annurev-arplant-042809-112226.
- Hashimoto M, Negi J, Young J, Israelsson M, Schroeder JI, Iba K. Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nat Cell Biol. 2006;8:391–397. doi:10.1038/ncb1387.
- Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Yamada M, Uchimiya H, Hashimoto M, Iba K. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature. 2008;452:483–486. doi:10.1038/nature06720.
- Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, Valerio G, Lamminmaki A, Brosche M, Moldau H, Desikan R, et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature. 2008;452:487–U15.
- Imes D, Mumm P, Böhm J, Al-Rasheid KAS, Marten I, Geiger D, Hedrich R. Open stomata 1 (OST1) kinase controls R–type anion channel QUAC1 in Arabidopsis guard cells. Plant J. 2013;74:372–382. doi:10.1111/tpj.12133.
- Hua D, Wang C, He J, Liao H, Duan Y, Zhu Z, Guo Y, Chen Z, Gong Z. A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell. 2012;24:2546–2561. doi:10.1105/tpc.112.100107.
- Geiger D, Maierhofer T, Al-Rasheid KA, Scherzer S, Mumm P, Liese A, Ache P, Wellmann C, Marten I, Grill E, et al. Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Sci Signal. 2011;4:ra32. doi:10.1126/scisignal.2001346.
- Zheng X, He K, Kleist T, Chen F, Luan S. Anion channel SLAH3 functions in nitrate-dependent alleviation of ammonium toxicity in Arabidopsis. Plant Cell Environ. 2015;38:474–486.
- Zhang A, Ren HM, Tan YQ, Qi GN, Yao FY, Wu GL, Yang LW, Hussain J, Sun SJ, Wang YF. S-type Anion Channels SLAC1 and SLAH3 Function as Essential Negative Regulators of Inward K+ Channels and Stomatal Opening in Arabidopsis. Plant Cell. 2016;28:949–955. doi:10.1105/tpc.16.01050.
- Mori IC, Murata Y, Yang Y, Munemasa S, Wang YF, Andreoli S, Tiriac H, Alonso JM, Harper JF, Ecker JR, et al. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca(2+)-permeable channels and stomatal closure. PLoS Biol. 2006;4:e327. doi:10.1371/journal.pbio.0040327.
- Latz A, Mehlmer N, Zapf S, Mueller TD, Wurzinger B, Pfister B, Csaszar E, Hedrich R, Teige M, Becker D. Salt stress triggers phosphorylation of the Arabidopsis vacuolar K+ channel TPK1 by calcium-dependent protein kinases (CDPKs). Mol Plant. 2013;6:1274–1289. doi:10.1093/mp/sss158.
- Lim CW, Lim S, Baek W, Lee SC. The pepper late embryogenesis abundant protein CaLEA1 acts in regulating abscisic acid signaling, drought and salt stress response. Physiol Plant. 2015;154:526–542. doi:10.1111/ppl.12298.