ABSTRACT
OsC2DP is a cytosolic protein containing a C2 domain recently identified in rice, which is translocated to the plasma membrane in response to salt stress. Here, we further investigated the subcellular localization of OsC2DP by truncation analysis. In consistent with OsC2DP, OsC2DP1−165 containing C2 domain at the N-terminus was localized to the cytosol. In contrast to OsC2DP1−165, OsC2DP166−290 lack of C2 domain at the C-terminus was localized to the cytosol and nucleus, which was similar to the GFP control. Under salt conditions, subcellular localization of both OsC2DP1−165 and OsC2DP166−290 was not altered and failed to migrate to plasma membrane. These results indicated that the subcellular localization was determined by C2 domain of OsC2DP under normal conditions and that both N- and C-terminus of OsC2DP are essential for its cytosol-plasma membrane translocation in response to salt stress.
A number of proteins containing lipid-binding domains have been identified in many organisms.Citation1,Citation2 Among them, C2-domain is one of the important lipid-binding modules with an affinity for both Ca2+ and lipids. The C2-domain, originally identified from the a, b, and c isoforms of mammalian Ca2+-dependent PKC1, contains approximately 130–145 amino acid residues.Citation3 C2 domain of PKCs is involved in the relocation of PKCs from the cytosol to the membrane by Ca2+-dependent phospholipid binding. In plants, a similar cytosol-plasma membrane translocation of C2 domain-containing proteins has been reported in rice such as OsERG1 and OsPBP1.Citation4,Citation5 However, some C2-domain proteins identified from plants were localized to the plasma membrane without translocation. But the deletion of C2 domain led to an alteration of its subcellular localization, which failed to target to the membrane and was localized to the cytoplasm such as CaSRC2 in pepper and Vr-PLC3 in mung bean.Citation6,Citation7 These reports suggested that C2 domain plays important roles in subcellular localization or membrane translocation of C2-domain proteins.
Recently, we identified a novel C2 domain protein OsC2DP in rice.Citation8 OsC2DP was localized to the cytosol. Under salt condition, OsC2DP was able to translocate to plasma membrane. Mutation of OsC2DP gene resulted in rice hypersensitivity to salt stress. Moreover, the expression level of some salt tolerance genes was indirectly regulated by OsC2DP, especially OsNHX4 and OsSOS1. These findings indicated that OsC2DP is required for salt tolerance in rice. In this report, we further investigated the subcellular localization of OsC2DP by truncation analysis. With the help of the protein domain prediction tool InterPro (https://www.ebi.ac.uk/interpro/beta/), we found two domains in the OsC2DP protein, one C2 domain at the N-terminus and a transmembrane domain at the C-terminus (). To determine the functional importance of these two domains in the OsC2DP localization,OsC2DP was truncated into OsC2DP1−165 (N-terminal amino acids 1 to 165) and OsC2DP166−290 (C-terminal amino acids 166 to 290) fused with GFP (). These constructs were transiently co-expressed with the cytosolic marker Ds-Red and nuclear marker Ghd7 in the rice protoplast cells by PEG-mediated transformation.Citation9 The green fluorescence of GFP-OsC2DP1−165 containing C2 domain was mainly co-localized with the cytosolic marker Ds-Red signal except for the nucleus in the protoplast cell (-L), which was consistent with the previous result of full-length OsC2DP.Citation8 Unlike GFP-OsC2DP1−165, GFP-OsC2DP166−290 lack of C2 domain was widely distributed at the cytoplasm and nucleus, which was similar to GFP alone (-D, M-T). These results indicated that C2 domain at the N-terminus of OsC2DP determined its subcellular localization.
Subsequently, to examine whether GFP-OsC2DP1−165 and GFP-OsC2DP166−290 are able to relocalize from the cytosol to the plasma membrane in response to salt stress, we co-expressed them with plasma membrane-localized marker mCherry-OsRac3Citation10 in the protoplast cells treated by 20 mM NaCl. As a result, the green fluorescence of both GFP-OsC2DP1−165 and GFP-OsC2DP166−290 was not overlapped with the mCherry-OsRac3 signal on the plasma membrane (), indicating that they failed to target to plasma membrane. Furthermore, their subcellular localizations were not altered, which were similar to those under normal conditions (). The results suggested that both N- and C-terminus of OsC2DP are required for its cytosol-plasma membrane translocation under salt stress.
Figure 1. Construction of truncated OsC2DP fusion with GFP protein. (A) Protein domains of OsC2DP. The gray rectangle indicates the full length of OsC2DP. The white rectangle in gray diagonal lines represents the C2 domain. The rectangle in blue represents the transmembrane domain. The red arrow indicates the truncation site. (B) Construction of GFP-OsC2DP1−165 and GFP-OsC2DP166−290 fusion protein.
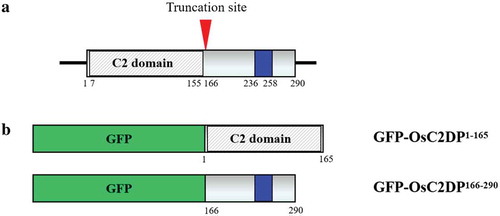
Figure 2. Subcellular localization of truncated OsC2DP under normal condition. (A-D) Co-expression of 35S::GFP and cytosolic marker Ds-Red (Red signal). (E-H, M-P) GFP-OsC2DP1−165 and GFP-OsC2DP166−290 fusion protein were co-expressed with Ds-Red, respectively. (I-L, Q-T) GFP-OsC2DP1−165 and GFP-OsC2DP166−290 were co-expressed with nuclear marker Ghd7 (Red signal), respectively. Scale bar = 10 μm.
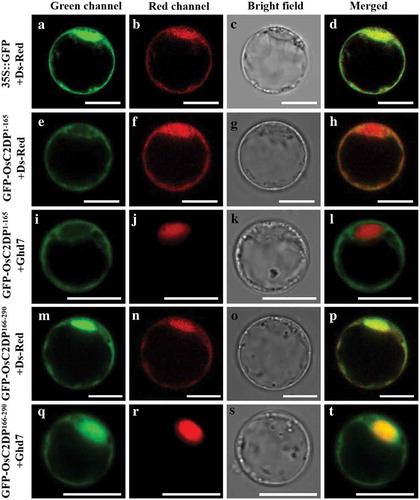
Figure 3. Subcellular localization of truncated OsC2DP under salt stress. After transformed by PEG solution, cells were incubated in W5 solution with the addition of 20 mM NaCl. Red fluorescence indicates the signal of plasma membrane marker mCherry-OsRac3. Scale bar = 10 μm.
In summary, our results demonstrated that the subcellular localization of OsC2DP was determined by its C2 domain at the N-terminus and that both N- and C-terminus of OsC2DP are involved in its translocation from the cytosol to plasma membrane in response to salt stress.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by Guangxi Natural Science Foundation (2016GXNSFFA380013), and the Project of High Level Innovation Team and Outstanding Scholar in Guangxi Colleges and Universities (2016).
Additional information
Funding
References
- Meijer HJ, Munnik T. Phospholipid-based signaling in plants. Annu Rev Plant Biol. 2003;54:1–3. PMID:14502992. doi:10.1146/annurev.arplant.54.031902.134748.
- Gallagher CM, Knoblich JA. The conserved C2 domain protein lethal (2) giant discs regulates protein trafficking in Drosophila. Dev Cell. 2006;11:641–653. PMID:17084357. doi:10.1016/j.devcel.2006.09.014.
- Nalefski EA, Falke JJ. The C2 domain calcium-binding motif: structural and functional diversity. Protein Sci. 1996;5:2375–2390. PMID:8976547. doi:10.1002/pro.5560051201.
- Kim CY, Koo YD, Jin JB, Moon BC, Kang CH, Kim ST, Park BO, Lee SY, Kim ML, Hwang I, et al. Rice C2-domain proteins are induced and translocated to the plasma membrane in response to a fungal elicitor. Biochemistry. 2003;42:11625–11633. PMID:14529272. doi:10.1021/bi034576n.
- Yang WQ, Lai Y, Li MN, Xu WY, Xue YB. A novel C2-domain phospholipid-binding protein, OsPBP1, is required for pollen fertility in rice. Mol Plant. 2008;1:770–785. PMID:19825580. doi:10.1093/mp/ssn035.
- Kim YC, Kim SY, Choi D, Ryu CM, Park JM. Molecular characterization of a pepper C2 domain-containing SRC2 protein implicated in resistance against host and non-host pathogens and abiotic stresses. Planta. 2008;227:1169–1179. PMID:18204857. doi:10.1007/s00425-007-0680-2.
- Kim YJ, Kim JE, Lee JH, Lee MH, Jung HW, Bahk YY, Hwang BK, Hwang I, Kim WT. The Vr-PLC3 gene encodes a putative plasma membrane-localized phosphoinositide-specific phospholipase C whose expression is induced by abiotic stress in mung bean (Vigna radiata L.). Febs Lett. 2004;556:127–136. PMID:14706839. doi:10.1016/S0014-5793(03)01388-7.
- Fu S, Fu L, Zhang X, Huang J, Yang G, Wang Z, Liu Y-G, Zhang G, Wu D, Xia J. OsC2DP, a novel C2 domain-containing protein is required for salt tolerance in rice. Plant Cell Physiol 2019; PMID:31198970. doi:10.1093/pcp/pcz115.
- Chen J, Liu Y, Ni J, Wang Y, Bai Y, Shi J, Gan J, Wu Z, Wu P. OsPHF1 regulates the plasma membrane localization of low- and high-affinity inorganic phosphate transporters and determines inorganic phosphate uptake and translocation in rice. Plant Physiol. 2011;157:269–278. PMID:21753117. doi:10.1104/pp.111.181669.
- Chen L, Shiotani K, Togashi T, Miki D, Aoyama M, Wong HL, Kawasaki T, Shimamoto K. Analysis of the Rac/Rop small GTPase family in rice: expression, subcellular localization and role in disease resistance. Plant Cell Physiol. 2010;51:585–595. PMID:20203239. doi:10.1093/pcp/pcq024.
