ABSTRACT
Plant roots show growth cessation as a primary response to mechanical stress. To clarify the molecular basis of this response, we have previously established an assay system to monitor the root growth response of Arabidopsis seedlings to mechanical stimuli using dialysis membrane-covered agar media. Here we examined the effect of plant hormones and their related molecules on this response. Amino-cyclopropane carboxylate, a precursor of ethylene, remarkably enhanced the growth reduction while silver ions, which block ethylene perception, nullified the response. Furthermore, salicylic acid, which inhibits ethylene biosynthesis, alleviated the root growth reduction, whereas methyl jasmonate had no apparent effect on the response. These results suggest that the root-growth cessation observed in response to mechanical stress involves ethylene signaling; however, this response may be independent from the pathway that integrates signals from ethylene and jasmonate.
KEYWORDS:
Plant organs sense and respond to mechanical stimuli during growth. Because roots grow toward gravity, they continually encounter obstacles in the soil, which leads to cessation of the growth, followed by restarting the growth in the direction that is possible. A pioneering study has revealed that, in the shoot of Arabidopsis, touch stimulation activates the expression of a subset of touch-induced genes, which encode calmodulin-related proteins and a xyloglucan endotransglycosylase.Citation1–Citation3 Mechanical signaling involves the rapid elevation of cytosolic Ca2+ concentration in plant cells.Citation4–Citation9 This process may be mediated by stretch-activated or voltage-gated ion channels located in the plasma membrane.Citation10 Ca2+ ions, in turn, activate downstream signaling pathways involving reactive oxygen speciesCitation11,Citation12 and plant hormones such as ethylene and auxin.Citation13–Citation15 However, the details of these downstream signaling events remain elusive mainly because few assay systems exist that allow monitoring of the growth response of the plant body to mechanical stress. To study in detail the molecular basis of the growth response to mechanical stress in roots, we generated a simple assay system using agar media covered with a dialysis membrane, which mimics mechanical stress because it prevents the roots from penetrating the agar media.Citation16
In our recent study, which identified a proton pump inhibitor, omeprazol, as a chemical compound that enhances the root-growth reduction in response to mechanical stress,Citation17 Arabidopsis seedlings were grown for 1 day after germination on vertically placed 0.8% (w/v) agar plates supplemented with half-strength Murashige and Skoog (MS) medium and 1% (w/v) sucrose; subsequently, the seedlings were transferred to plates that were supplemented with the test compound and covered with a dialysis membrane (12,000–14,000 MWCO, Spectra/Por 4, Spectrum Laboratories). The plates were incubated horizontally or vertically for 2 days and the growth of primary roots in each plate was measured using Image J. In the current study, to fully assess the effect of chemicals on the root-growth response to mechanical stimuli, one-day-old seedlings were transferred to dialysis-membrane-covered plates with each test compound and once incubated vertically for 1 day. They were then subjected to vertical or horizontal growth for further 2 days in the presence of chemicals.
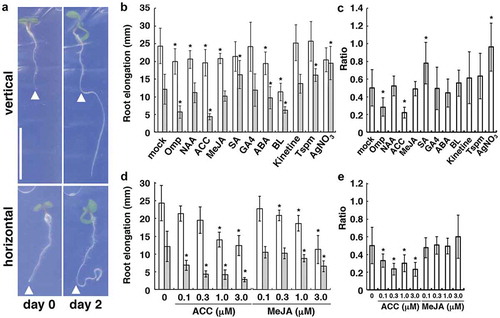
In the medium with no test compound, the roots of Arabidopsis seedlings showed an approximately 2-fold reduction in net growth on horizontal plates compared with vertical ones ()). We first confirmed that omeprazole obviously enhanced the growth reduction (,)) in a way that was similar to the case of simultaneous treatment with omeprazole and mechanical stress.Citation17 Given that omeprazole acts as an inhibitor of Ca2+ or H+ pumps, it may cause a delay in the recovery from cessation of growth. We then tested a series of plant growth regulators and found that 300 nM of amino-cyclopropane carboxylate (ACC; used as a precursor of ethylene) resulted in a severe growth reduction of the roots, while salicylic acid (SA) alleviated the growth reduction during horizontal growth (,)). Other plant-growth regulators, including an artificial auxin, naphthaleneacetic acid, methyl jasmonate (MeJA), gibberellin A4, abscisic acid, brassinolide, kinetine, and thermospermine, had no significant effect on the root-growth reduction (,)). The concentrations of these plant-growth regulators tested here were based on those used in many studies, while different concentrations of ACC and MeJA were further examined. ACC had an enhancing effect on the root-growth reduction at concentrations ranging from 0.1 to 3 µM although increased ACC concentrations were inhibitory of the growth itself (,)). These concentrations of ACC are consistent with those used in a previous study on the response of Arabidopsis roots to ethylene.Citation18 Although MeJA was also inhibitory of the growth itself at micromolar levels, as shown in previously,Citation19 lower concentrations of MeJA were shown to have no apparent effect on the ratio of horizontal vs. vertical root growth (,)). We also examined the effect of silver ions, which block the binding of ethylene to its receptor, on the growth response to mechanical stress and confirmed that they almost nullified the response (,)).
Figure 1. Effect of plant growth regulators on the root growth under mechanical stress. (a) effect of a dialysis membrane (as a mechanical barrier) on root growth. One-day-old seedlings were transferred onto dialysis-membrane-covered agar plates that were placed vertically or horizontally (day 0) and grown for 2 days (day 2). The arrowheads indicate the position of the root tip at day 0. Bars, 10 mm. (b) effect of omeprazol (OMP), plant growth regulators, and the related compounds on root growth after transfer to vertically (white bars) and horizontally (gray bars) placed plates, as shown in (a). Each chemical (3 µM OMP, 30 nM naphthaleneacetic acid (NAA), 300 nM ACC, 300 nM MeJA, 1 µM gibberellin A4 (GA4), 1 µM abscisic acid (ABA), 1 nM brassinolide (BL), 100 nM kinetine, 30 µM thermospermine (Tspm), and 10 µM AgNO3) was supplemented continuously after germination. (c) Ratio of horizontal to vertical root growth shown in (b). (d) effect of different concentrations of ACC and MeJA on the root growth under mechanical stress. (e) ratio of horizontal to vertical root growth shown in (d). In (b) to (e), the error bars represent SD (n > 20). The asterisks indicate statistically significant differences between means, as assessed using Student’s t-test (P < .01).
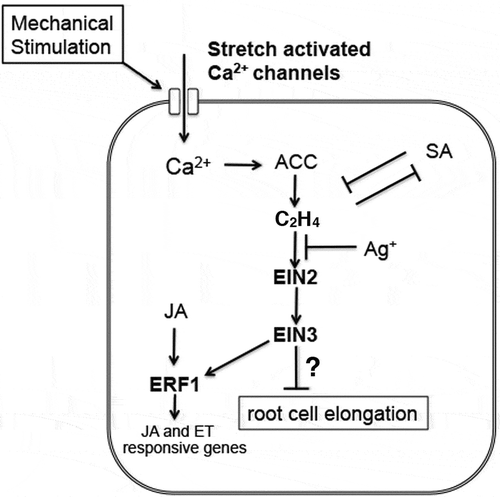
In summary, among other plant growth regulators, ethylene plays a central role in the mechanical-stress-induced root-growth cessation. A simplified hypothetical model of the signaling pathway underlying this response in Arabidopsis is shown in . However, some caution is warranted because hormones or chemicals may have varying effects. A study indicates that silver ions can promote auxin efflux independently of the inhibitory effect on ethylene perception.Citation20 SA was shown to alleviate the growth cessation effectively. This might be attributable to the inhibitory effect of SA on ethylene production from ACC. Other studies reported that SA inhibits ethylene biosynthesis in some plant species.Citation21,Citation22 However, given the diverse effects of SA on the plant stress response, other possibilities should also be considered. There is also increasing evidence indicating that the SA- and ethylene/JA-mediated pathways are mutually antagonistic in the defense response.Citation23 Conversely, other plant growth regulators that are implicated in mechanical-stress signaling, including MeJACitation24 and gibberellin,Citation25 had no apparent effect on the root growth response. The hierarchical relationship between an ER membrane-localized ethylene signal mediator, ETHYLENE INSENSITIVE2 (EIN2), and two transcription factors, EIN3 and ETHYLENE RESPONSE FACTOR1 (ERF1), has been reported in previous studies.Citation26 Because signals from JA and ethylene are integrated into ERF1 during the defense response,Citation27 the mechanical-stress signaling addressed here may be uncoupled from the function of ERF1. To date, we have shown that a loss-of-function mutant of the EIN2 gene displays no root-growth cessation in this assay system.Citation17 Further mutant analyzes will help identify all of the molecules that participate in this signaling pathway.
Disclosure of potential conflicts of interest
The authors declare no potential conflicts of interest.
Additional information
Funding
References
- Braam J, Davis RW. Rain-, wind-, and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell. 1990;60:1–3. doi:10.1016/0092-8674(90)90587-5.
- Sistrunk ML, Antosiewicz DM, Purugganan MM, Arabidopsis BJ. TCH3 encodes a novel Ca2+ binding protein and shown environmentally induced and tissue-specific regulation. Plant Cell. 1994;6:1553–1565. doi:10.1105/tpc.6.11.1153.
- Lee D, Polisensky DH, Braam J. Genome-wide identification of touch- and darkness-regulated Arabidopsis genes: a focus on calmodulin-like and XTH genes. New Phytol. 2005;165:429–444. doi:10.1111/j.1469-8137.2004.01238.x.
- Knight MR, Campbell AK, Smith SM, Trewavas AJ. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature. 1991;352:524–526. doi:10.1038/352524a0.
- Trewavas A, Knight M. Mechanical signalling, calcium and plant form. Plant Mol Biol. 1994;26:1329–1341. doi:10.1007/BF00016478.
- Legue V, Blancaflor E, Wymer C, Perbal G, Fantin D, Gilroy S. Cytoplasmic free Ca2+ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol. 1997;114:789–800. doi:10.1104/pp.114.3.789.
- Fasano JM, Massa GD, Gilroy S. Ionic signaling in plant responses to gravity and touch. J Plant Growth Regul. 2002;21:71–88. doi:10.1007/s003440010049.
- Braam J. In touch: plant responses to mechanical stimuli. New Phytol. 2005;165:373–389. doi:10.1111/j.1469-8137.2004.01263.x.
- Kurusu T, Kuchitsu K, Nakano M, Nakayama Y, Iida H. Plant mechanosensing and Ca2+ transport. Trends Plant Sci. 2013;18:227–233. doi:10.1016/j.tplants.2012.12.002.
- Nakagawa Y, Katagiri T, Shinozaki K, Qi Z, Tatsumi H, Furuichi T, Kishigami A, Sokabe M, Kojima I, Sato S, et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc Natl Acad Sci USA. 2007;104:3639–3644. doi:10.1073/pnas.0607703104.
- Monshausen GB, Bibikova TN, Weisenseel MH, Gilroy S. Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. Plant Cell. 2009;21:2341–2356. doi:10.1105/tpc.109.068395.
- Monshausen GB, Haswell ES. A force of nature: molecular mechanisms of mechanoperception in plants. J Exp Bot. 2013;64:4663–4680. doi:10.1093/jxb/ert204.
- Biro RL, Jaffe MJ. Thigmomorphogenesis: ethylene evolution and its role in the changes observed in mechanically perturbed bean plants. Physiol Plant. 1984;62:289–296. doi:10.1111/ppl.1984.62.issue-3.
- Chehab EW, Eich E, Braam J. Thigmomorphogenesis: a complex plant response to mechano-stimulation. J Exp Bot. 2009;60:43–56. doi:10.1093/jxb/ern315.
- Sassi M, Traas J. When biochemistry meets mechanics: a systems view of growth control in plants. Curr Opin Plant Biol. 2015;28:137–143. doi:10.1016/j.pbi.2015.10.005.
- Okamoto T, Tsurumi S, Shibasaki K, Obana Y, Takaji H, Oono Y, Rahman A. Genetic dissection of hormonal responses in the roots of Arabidopsis grown under continuous mechanical impedance. Plant Physiol. 2008;146:1651–1662. doi:10.1104/pp.107.115519.
- Okamoto T, Takatani S, Noutoshi Y, Motose H, Takahashi T. Omeprazole enhances mechanical stress-induced root growth reduction in Arabidopsis thaliana. Plant Cell Physiol. 2018;59:1581–1591. doi:10.1093/pcp/pcy131.
- Le J, Vandenbussche F, Van Der Straeten D, Verbelen JP. In the early response of Arabidopsis roots to ethylene, cell elongation is up- and down-regulated and uncoupled from differentiation. Plant Physiol. 2001;125:519–522. doi:10.1104/pp.125.2.519.
- Staswick PE, Su W, Howell SH. Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc Natl Acad Sci USA. 1992;89:6837–6840. doi:10.1073/pnas.89.15.6837.
- Strader LC, Beisner ER, Bartel B. Silver ions increase auxin efflux independently of effects on ethylene response. Plant Cell. 2009;21:3585–3590. doi:10.1105/tpc.108.065185.
- Leslie CA, Romani RJ. Inhibition of ethylene biosynthesis by salicylic acid: kinetic and comparative aspects. Plant Physiol. 1988;88:833–837. doi:10.1104/pp.88.3.833.
- Romani RJ, Hess BM, Leslie CA. Salicylic acid inhibition of ethylene production by apple discs and other plant tissues. J Plant Growth Regul. 1989;8:63–69. doi:10.1007/BF02024927.
- Li N, Han X, Feng D, Yuan D, Huang LJ. Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: do we understand what they are whispering? Int J Mol Sci. 2019;20:671. doi:10.3390/ijms20030671.
- Chehab EW, Yao C, Henderson Z, Kim S, Braam J. Arabidopsis touch-induced morphogenesis is jasmonate mediated and protects against pests. Curr Biol. 2012;22:701–706. doi:10.1016/j.cub.2012.02.061.
- Lange MJ, Lange T. Touch-induced changes in Arabidopsis morphology dependent on gibberellin breakdown. Nat Plants. 2015;1:14025. doi:10.1038/nplants.2014.25.
- Stepanova AN, Alonso JM. Ethylene signaling and response: where different regulatory modules meet. Curr Opin Plant Biol. 2009;12:548–555. doi:10.1016/j.pbi.2009.07.009.
- Lorenzo O, Piqueras R, Sanchez-Serrano JJ, Solano R. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell. 2003;15:165–178. doi:10.1105/tpc.007468.