ABSTRACT
Studies on UV-B-induced plant photomorphogenesis mainly focus on Arabidopsis shoots (hypocotyl, leaf, petiole, and stem) but less on roots. In the present research, the low-level UV-B (0.2 W·m−2) induced a decrease in the number of root cells in the meristem zone and an inhibition of the cell length in the maturation zone of roots in Arabidopsis thaliana L.Heynh (Col-0). UV-B-induced root growth inhibition was recovered by the addition of GA3 to culture media. GA3 played an important role in UV-B-induced inhibition of root growth. The cop1-4 mutant with more meristem cell and longer mature cells exhibited longer root length under low-level UV-B. COP1 acted as a positive regulator of root growth under UV-B, through regulation of cell division and elongation. The sto mutant exhibited a shorter root length under UV-B with similar cell length but fewer meristem cells compared with wild type (Col-0). STO only regulated cell division, but cell expansion was not affected. UV-B radiation also inhibited the root growth of uvr8 mutant, and the degree of inhibition was greater than for wild type (Ler). UV-B inhibited the growth of Arabidopsis root, possibly because it changes the GA signal and inhibited cell division and cell elongation, which be related to COP1 and STO genes.
1. Introduction
Ultraviolet light is an integral part of the sunlight necessary for plant growth. In addition to being partly absorbed by the ozone layer, medium-wave ultraviolet radiation (UV-B, 280–315 nm) reaches the ground directly and, due to its high energy, has important effects on plants and animals on the earth’s surface.Citation1
UV-B radiation induces two different responses in plants, including stress response and photopoietic response. The difference between the two reactions is mainly based on the level of UV-B radiation, treatment time, the wavelength of the light source, and whether plants have adapted to UV-B radiation.Citation2 The effects of UV-B radiation on plants are mainly divided into the following three aspects: molecular, biochemical, and physiological aspects.Citation3 High-level UV-B radiation causes stress response and damage to plants, including damage to DNA, proteins, and cytoplasmic membranes, production of reactive oxygen species (ROS), cell cycle arrest, inhibition of photosynthesis, and various cell processes.Citation2–Citation6 In contrast, on the positive side, low-level UV-B radiation promotes photomorphogenetic response of plants, such as inhibiting the growth of hypocotyl, leading to shortened internodes, reduced plant branches, thickened leaf epidermis, stimulating cotyledon opening, stomatal opening, and promoting the synthesis of anthocyanins and flavonoids.Citation2,Citation5,Citation7–Citation9 UV-B radiation caused phototropism of plants by affecting auxin signals.Citation10 Recent studies have found that in a low-red light/far-red light environment, UV-B inhibits the transcription of GA3 synthesis genes, stabilizes DELLA protein, and inhibits the synthesis and signal pathway of auxin, thus inhibiting the shade-avoidance reaction of plants.Citation11 The effects of elevated UV-B on root growth of soybean seedlings were regulated by signaling molecules, and the degree of the effects was related to the intensity of UV-B radiation.Citation12
Light plays an important role in the growth and development of plants, and too much light also causes serious damage to plant cells.Citation13 During the evolution of terrestrial plants, roots grew in the dark underground and stems grew in the light during the day. Eventually, the root adapts to growing in the dark, and the stem grows toward the light. In previous studies, Arabidopsis grown in a laboratory in a glass dish had roots growing in the light environment.Citation13 Moreover, the stress response induced by high-level UV-B radiation inhibited the morphogenetic response induced by low-level UV-B radiation.Citation14
Plant hormones, including gibberellin (GA), cytokinin, auxin (IAA), abscisic acid (ABA), ethylene, and brassinolide (BR), play an important role in plant growth, aging, dormancy, and stress resistance. The role of GA is to accelerate the elongation of cells, promote cell division, and promote cell expansion. GA also promotes the proliferation of Arabidopsis cells, and this process was also achieved through the destruction of DELLA protein.Citation15 IAA promotes the formation of lateral root primordia.Citation16 In Arabidopsis, the application of cytokinin reduces the size of the partial root zone and does not affect the division potential of meristem cells but promotes the differentiation of transition zone cells.Citation17 Cytokinin inhibits the formation of lateral roots, and reducing the content of cytokinin or blocking its signaling pathway greatly increases the number of lateral roots.Citation18 ABCG14 (ATP-binding cassette transporter subfamily G14) transports cytokinin from the root to the above-ground part of Arabidopsis.Citation19
Different wavelength bands of UV-B cause the expression of different plant genes: short-wave causes the expression of stress-related genes, while long-wave mainly causes the expression of UV-B light morphogenesis-related genes.Citation20 The photoreceptors found in plants to date include phyA-E, a photosensitive pigment that senses red and far-red light, phot1 and phot2; two cryptochrome cry1 and cry2; ZTL, FKF1, and LKP2, members of the Zeitlupe family; and UVR8 (UV Resistance Locus 8), a receptor that is sensitive to UV-B.Citation6,Citation21,Citation22 UVR8 was first obtained by screening Arabidopsis mutants susceptible to UV-B.Citation8 Subsequent studies identified many alleles of uvr8 mutants, demonstrating that UVR8 was the first component of the UV-B signaling pathway and was necessary for the photomorphogenetic pathway of UV-B.Citation23,Citation24 Transcriptome analysis revealed that UVR8 regulates a series of genes that play an important role in UV protection and repair of UV damage.Citation23 UVR8 functions alone in UV-B signal transduction and needs to interact with other proteins, including COP1, RUP1, and RUP2. Although the function of UVR8 in the cytoplasm is ruled out at present, the vast majority of evidence indicates that its important role in UV-B signaling is mainly carried out in the nucleus.Citation25 COP1 protein contains three functional domains: ring zinc finger binding domain, curly spiral domain, and WD40 repeat domain.Citation26 COP1 plays an important role in the signal pathway of visible light and is an important negative regulator of light morphogenesis of seedlings.Citation27 New functions of Arabidopsis COP1 protein in UV-B response have been identified.Citation28 The cop1 seedlings showed extremely light morphogenesis and dwarf phenotypes under light but did not show typical morphogenetic response and molecular response in UV-B.Citation28 Because the hypocotyl of cop1 seedlings is very short under white light, they did not demonstrate shorter phenotypes than under white light after UV-B irradiation.Citation28 The cop1 mutants did not produce phenotypes of UV-B inhibiting hypocotyl growth, the accumulation of flavonoids and HY5 caused by UV-B or changes in gene expression, so COP1 was viewed as a positive regulator in UV-B radiation response.Citation28 STO is a B-box zinc finger protein. STO is a negative regulator in the UV-B signaling pathway.Citation29 In other studies, UV-B promoted the expression of STO, and sto mutants showed sensitive phenotype to UV-B, extreme short stature, shortened hypocotyl, and increased anthocyanin accumulation.Citation29 Therefore, STO is considered to be a negative regulator in the UV-B signaling pathway.
So far, there are few studies on the inhibition of plant root growth by UV-B. It is not clear how UV-B inhibits the root growth of Arabidopsis and whether UV-B photoreceptor UVR8 plays a role in this process. In the present study, Arabidopsis and a series of related mutants were used as the research material. Based on the fact that most of Arabidopsis planted in the laboratory were exposed to light, the reason and mechanism of low-level UV-B inhibiting root growth of Arabidopsis were discussed. The lack of research on UV-B inhibiting root growth of Arabidopsis was supplemented, so as to further improve the signal pathway of UV-B.
2. Materials and methods
2.1. Plant materials
Arabidopsis thaliana L.Heynh (Col-0) and its mutants sto and cop1-4 and Landsberg erecta (Ler) and its mutant uvr8-2 were used in the present study. Arabidopsis seeds were vernalized for 3 d at 4°C in dark, after disinfection by 10% sodium hypochlorite solution, and grown vertically on 1/2 MS culture medium (containing 1% sucrose, 0.8% agar, pH 5.8). The temperature was approximately 22°C, and the photosynthetic active radiation intensity was 10 µmol m−Citation2s−Citation1 (as measured by Quantithern Light Meter, Hansatech, UK).
Ultraviolet light (UV-313, Beijing Institute of Photoelectric) was filtered by cellulose diacetate film (the long pass filter type, cutoff at 292 nm) for the UV-B radiation and by Mylar film (the long pass filter type, cutoff at 315 nm) for UV-A light as control. UV-B spectral irradiance was calibrated to 300 nm by weighted calculation of the plant response spectrum to obtain biological effective irradiance (UV-BBE).Citation30 The low-level UV-B used in this study was 0.2 W·m−Citation2, while the high-level UV-B was 0.6 W·m−Citation2.
Seeds were given a continuous low level of UV-B radiation for 6 d, with 10 µmol m−Citation2s−Citation1 white light. A number of treatments and measurements were undertaken as below.
2.2. Determination of root length
The germination of seeds and the growth of seedlings were observed daily for 6 d after sowing. The root length of seedlings was measured by Image J software. The experiments include three technical replicates and three biological replicates.
2.3. The number of cell division and cell size
The seedlings of Arabidopsis (Col-0) exposed to white light and white light with low-level UV-B for 6 d were used. 3 mg L−Citation1 PI fluorescent dye (Sigma) was added to a clean slide. The roots were divided into three zones: the meristem zone, the elongation zone, and the maturation zone. The most distal parts of the root apex after PI staining are active meristem zone tissues. The maturation zone is identified by external clusters of root hairs. The elongation zone lies between the meristem and maturation zones. The cell number in the root meristem was obtained by counting cortex cells from the quiescent center to the cell showing no signs of rapid elongation, and the elongation/differentiation zone was specified from the first cortex cell that exited from the meristem.Citation31 Root length was measured from digital images of the plates using Image J software. The roots of Arabidopsis seedlings were placed on the dye solution. The roots were covered with a cover glass and placed in the dark for 3 minutes. The samples were then observed and photographed under a confocal laser microscope. The experiments include three technical replicates and three biological replicates.
2.4. Hormone treatment
IAA (indole-3-acetic acid, indol-yl-3-acetic acid), GA3 (gibberellin A3), and 6-BA (6-benzylaminopurine) were added to the 1/2 MS medium, respectively. Each hormone had three concentrations, including 0.1, 1, and 10 µM. Arabidopsis wild-type Col-0 seeds were seeded on the medium in the petri dishes containing hormone and the medium without hormone and maintained under white light and white light with low-level UV-B for 6 d, respectively. The root length of the seedlings was measured on the pictures in computer by Image J software. The experiments include three technical replicates and three biological replicates.
2.5. Different genotypes
Arabidopsis wild type (Col-0), sto, cop1-4, and uvr8-2 mutant seeds were seeded on the 1/2 MS medium in the petri dishes under white light and white light with low-level UV-B for 6 d, respectively. The root length of the seedlings was measured on the pictures in computer by Image J software. The experiments include three technical replicates and three biological replicates.
2.6. Data analysis
The GraphPad Prism 7.0 was used for statistical analysis and drawing. For comparing the results of different treatments, variance analysis is followed by a post-hoc test in order to determine pairwise differences. Differences were considered significant for P < 0.05.
3. Results
3.1. Effects of UV-B radiation on the root growth of Arabidopsis seedlings
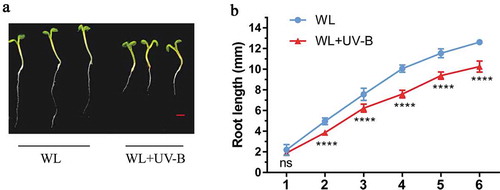
The root growth of Arabidopsis seedlings under white light and white light with low-level UV-B is shown in . The result showed that the root length of seedlings under white light was significantly (P = 0.0056, paired t test) longer than those of Arabidopsis seedlings under white light with low-level UV-B (,b)). The results showed that low-level UV-B (0.2 W•m−2) inhibited the growth of hypocotyl and root of Arabidopsis seedlings.
Figure 1. UV-B inhibited the root growth of Arabidopsis. Wild type (Col-0) was grown under continuous white light or white light with UV-B for 6 d. (a) UV-B inhibition of the root growth of Arabidopsis seedlings; (b) the root length of Arabidopsis under white light or white light with UV-B; WL: White light; WL+UV-B: White light with UV-B, scale bar = 1 mm. Data are expressed as mean values ± standard errors from three replicates, and error bars represent standard errors. The symbol ‘*’ indicates statistical difference P < 0.05, and the symbol ‘****’ indicates statistical difference P < 0.0001 (paired t test, P < 0.05).
3.2. UV-B radiation inhibited root cell division and cell elongation in Arabidopsis
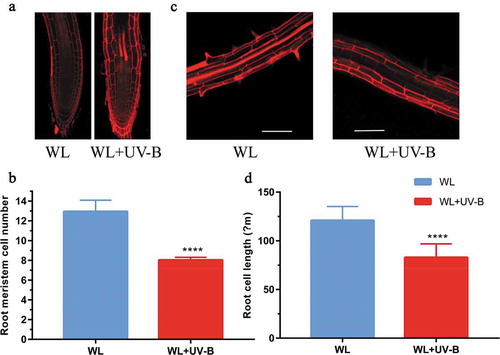
The length of the root is determined by the size and number of cells.Citation32 In the root, the cells in the meristem zone are small, but they gradually grow longer through the elongation zone until they reach their maximum length in the mature zone. The results showed that the cell number in root meristem zone of Arabidopsis under white light was significantly(P < 0.0001, paired t test) higher than that under white light with low-level UV-B, and the cell length in the root maturation zone of Arabidopsis growing under white light was significantly (P < 0.0001, paired t test) longer than that under white light with low-level UV-B (,b)). These results indicated that UV-B inhibited the root growth of Arabidopsis through cell division and cell elongation.
Figure 2. UV-B radiation inhibited cell division and cell length. Wild type (Col-0) was grown under continuous white light or white light with UV-B for 6 d. (a) The meristem zone and maturation zone of roots were stained by PI and then visualized by microscopically. (b) The average meristem cell number and cell length of the maturation zone were measured by Image J software, scale bar = 100 μm. Data are expressed as mean values ± standard errors from three replicates, and error bars represent standard errors. The symbol ‘*’ indicates statistical difference P < 0.05 and the symbol ‘****’ indicates statistical difference P < 0.0001 (paired t test, P < 0.05).
3.3. Effects of hormone treatment on root growth under UV-B environment
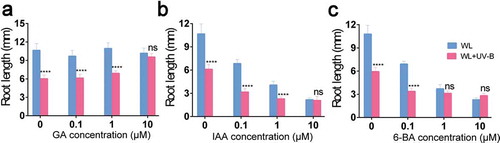
Different concentrations of GA3 had no significant effect on the root growth of Arabidopsis under white light ()). However, the root length of Arabidopsis significantly increased with the increase of GA3 concentration under the low-level UV-B. But there was no significant difference between the root length of Arabidopsis under low-level UV-B and white light at 10 μM GA3. With the different concentrations of IAA ()) and 6-BA ()), the root length of Arabidopsis under both white light and low-level UV-B gradually shortened with the increase in hormone concentration, and there was no significant (IAA, P = 0.0855, paired t test; 6-BA, P = 0.1912, paired t test) difference between control and UV-B treatment. Thus, the response of Arabidopsis to IAA and 6-BA under UV-B did not change compared with that under white light. Based on the above results, it is concluded that UV-B inhibits the growth of seedling roots of Arabidopsis probably because UV-B inhibits the response of Arabidopsis to GA3, and the root length of Arabidopsis in the culture medium supplemented with GA3 increases with the increase of hormone concentration.
Figure 3. Effect of hormones on Arabidopsis root growth. Wild type (Col-0) was grown under continuous white light or white light with UV-B for 6 d. (a) The root length of Arabidopsis with the treatment of GA. (b) The root length of Arabidopsis with the treatment of IAA. (c) The root length of Arabidopsis with the treatment of 6-BA. Data are expressed as mean values ± standard errors from three replicates, and error bars represent standard errors. The symbol ‘*’ indicates statistical difference P < 0.05 and the symbol ‘****’ indicates statistical difference P < 0.0001 (paired t test, P < 0.05).
3.4. COP1 and STO genes affect root growth under UV-B radiation
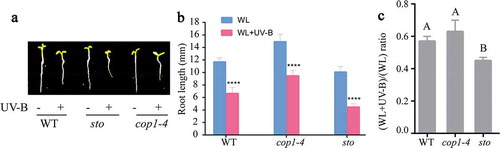
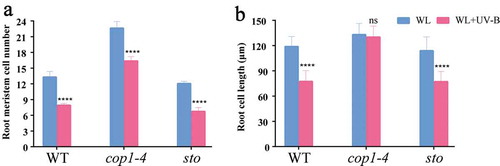
As shown in ,b), there was a significant difference in the root length between the wild-type and cop1-4 mutants growing under white light and low-level UV-B, and the root length of sto mutants growing was significantly shorter than that of the wild-type under white light and low-level UV-B. From ), the ratio (WL+UV-B)/(WL) of root length in WT is 57%, the ratio (WL+UV-B)/(WL) of root length in cop1 is 63%, and the ratio (WL+UV-B)/(WL) of root length in sto is 45%. The results indicated that the elongation of the roots of sto mutants would be inhibited under low-level UV-B radiation, while the elongation of the roots of cop1-4 mutants would be promoted.
Figure 4. The primary root length of sto and cop1-4 mutants under UV-B radiation. WT (Col-0), cop1-4, and sto mutants were grown under continuous white light or white light with UV-B for 6 d. (a) Phenotype of primary root length of wild type (Col-0), sto, and cop1-4 mutant. (b) Bar graph showing the primary root length measured by Image J software, scale bar = 1 mm. (c) The ratio (WL+UV-B)/(WL) of root length. Data are expressed as mean values ± standard errors from three replicates, and error bars represent standard errors. The symbol ‘*’ indicates statistical difference P < 0.05 and the symbol ‘****’ indicates statistical difference P < 0.0001 (two-way ANOVA, Turkey’s multiple comparisons test, P < 0.05).
3.5. Roles of COP1 and STO genes in root cell division and cell elongation
The cell division ability of wild-type roots under white light was weaker than cop1-4 mutants, and the cell division ability of wild-type roots under white light was stronger than sto mutants.
UV-B inhibits root growth in Arabidopsis because it inhibits root cell elongation and division (). As shown in ,b), cell division number and cell size under white light were significantly larger (P < 0.0001, two-way ANOVA, Tukey’s multiple comparisons test) than those under low-level UV-B. As for the sto mutants, cell division number and cell size under white light were significantly smaller (P < 0.0001, two-way ANOVA, Tukey’s multiple comparisons test) than those under low-level UV-B. The cop1-4 mutants showed significantly (P < 0.0001, two-way ANOVA, Tukey’s multiple comparisons test) less inhibition of cell division and increased cell size than wild-type mutants under UV-B radiation. The sto mutants showed significant (P = 0.0455, two-way ANOVA, Tukey’s multiple comparisons test) inhibition of cell division than the wild-type mutants under UV-B radiation. These results suggest that COP1 gene is a positive regulator of UV-B inhibiting cell division and cell size, and STO gene is a negative regulator of UV-B inhibiting cell division.
Figure 5. The function of COP1 and STO genes on root cell division and cell length. WT (Col-0), cop1-4, and sto mutants were grown under continuous white light or white light with UV-B for 6 d. Cell number and cell length were measured by ImageJ software. (a) Cell number in meristem zone of root; (b) cell length in mature zone of the root. Data are expressed as mean values ± standard errors from three replicates, and error bars represent standard errors. The symbol ‘*’ indicates statistical difference P < 0.05 and the symbol ‘****’ indicates statistical difference P < 0.0001 (two-way ANOVA, Turkey’s multiple comparisons test).
3.6. UV-B radiation inhibits the root growth of Arabidopsis without the light receptor UVR8
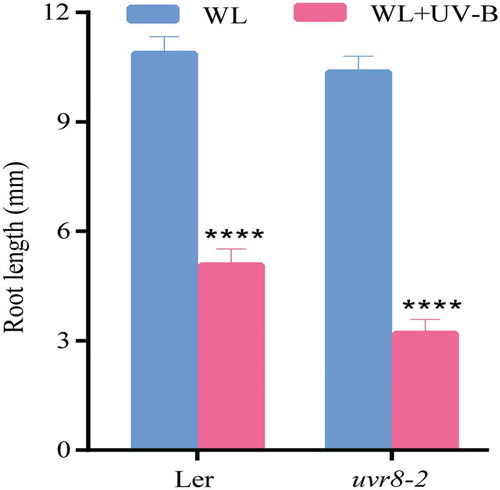
As shown in , the root length of wild-type (Ler) and that of uvr8-2 mutant was inhibited, after UV-B irradiation, by 55% and 69%, respectively. However, there was no significant difference between wild-type (Ler) and uvr8-2 mutant under white light. These results indicated that UVR8 did not act as a photoreceptor during the inhibition of UV-B on the root growth of Arabidopsis.
Figure 6. UV-B-induced root growth inhibition. Wild type (Ler) and uvr8-2 were grown under continuous white light or white light with UV-B for 6 d. The root length was measured by Image J software. Data are expressed as mean values ± standard errors from three replicates, and error bars represent standard errors. The symbol ‘*’ indicates statistical difference P < 0.05 and the symbol ‘****’ indicates statistical difference P < 0.0001 (two-way ANOVA, Turkey’s multiple comparisons test).
4. Discussion
4.1. Low-level UV-B inhibited the root growth of Arabidopsis
UV-B is an intrinsic component of sunlight and an important environmental factor. Studies on the effects of UV-B on plant growth mainly focus on aboveground parts, including hypocotyl, leaves, and secondary metabolites. There is little research on the roots that grow underground. In the present experiment, low-level UV-B was used to treat Arabidopsis seedlings, and it was found that UV-B did not only inhibit the hypocotyl growth but also inhibit the root growth ()). After seed germination, root growth of Arabidopsis in the UV-B treatment group was slower than that in the control group ()). The study of JiangCitation29 also showed that UV-B (0.6 W·m−2) indeed inhibited the root growth of Arabidopsis, although the level of UV-B (0.3 W·m−2) was used in the present experiment.
Plant growth mainly occurs through the elongation of plant cells and cell division. PI was used to stain plant roots in the present study, and it was found that UV-B did not only inhibit cell elongation but also inhibit cell division (). This suggests that UV-B inhibits the growth of Arabidopsis, probably because UV-B affects the expression of genes related to the cell cycle and cell elongation.
4.2. Effects of hormones on the root growth of Arabidopsis under low-level UV-B
The effects of hormones on plant growth have been extensively studied. In recent years, the interaction between UV-B and hormones has also been discussed. In the present study, Arabidopsis growing under white light and UV-B showed consistent responses to IAA and 6-BA, both of which inhibited the root growth of Arabidopsis. With the increase of hormone concentration, the degree of inhibition became more obvious (paired t test) (,c)). These results indicated that the root response to IAA and cytokinin under the UV-B was the same as that of Arabidopsis under white light. However, other studies have shown that UVR8 responds to the low levels of UV-B and strongly inhibits the elongation of the hypocotyl by inhibiting the synthesis of IAA and cell elongation.Citation11 In the present experiment, UV-B inhibits the root growth of Arabidopsis without the participation of UVR8 (). So it did not change the auxin signal in the root through UVR8. Studies have shown that cytokinin inhibits root elongation and branching.Citation33 Cytokinin is a negative regulator of root growth, Citation34 and the present results ()) showed that Arabidopsis grown on medium supplemented with cytokinin had shorter roots than those grown on normal medium. However, different concentrations of GA have no significant effect on Arabidopsis growing under white light but have a promoting effect on Arabidopsis growing under UV-B. When GA concentration reached 10 μM, the root length of Arabidopsis under UV-B treatment was the same as that of Arabidopsis growing under white light ()). This suggests that UV-B inhibits the growth of Arabidopsis root, probably because UV-B inhibits GA synthesis or transport-related gene expression. Studies have shown that UVR8 is sensitive to UV-B, thereby promoting the expression of GA2ox1 and thus inactivating GA. Meanwhile, UV-B inhibits the transcription of GA20ox2, a synthetic GA gene, and ultimately inhibits the growth of hypocotyls in Arabidopsis.Citation11 This is the possible reason why UV-B also inhibits the root growth.
4.3. Effects of COP1 and STO on the root growth of Arabidopsis
COP1 is involved in both optical signals and UV-B signals. It is a positive regulator in the UV-B signaling pathway, Citation28 promoting the expression of genes in a series of UV-B signaling pathways. However, its role in UV-B inhibition of root growth in Arabidopsis remains unknown. In the present study, cop1-4 mutants growing for 6 d showed longer primary roots under the UV-B environment than the WT ()), indicating that COP1 plays an important role in the growth of Arabidopsis root under UV-B environment. Light promoted root elongation in Arabidopsis by affecting the cytoskeletal structure of actin through COP1.Citation35 COP1 affected the root growth of Arabidopsis by regulating IAA transport, and cop1-4 mutants show a larger meristem zone than WT root in both dark and light.Citation36 The cop1-4 mutant in this study also showed a greater cell division number than WT in both white light and UV-B ()). The cop1-4 and WT have similar cell sizes in white light, but the cells of the cop1-4 mutant are significantly smaller than those of the wild type (Col-0) in the UV-B environment ()). These results suggested that COP1 affected root growth by affecting cell division and cell elongation. STO is a negative regulator in red, far-red, blue, and UV-B signaling pathways.Citation29,Citation37,Citation38 In this study, as shown in , there was no significant difference in taproot length between sto and WT in the white light environment, but under the UV-B environment, sto mutants showed shorter taproot length. This is consistent with the research results of Jiang.Citation29 The length of cells in the mature root region in the sto mutant did not differ from that in the WT under the UV-B environment, but the cell division number in the sto mutant was less than that in the WT under the UV-B environment in the present study. The results suggested that STO affected root growth by inhibiting cell division.
4.4. Effects of UV-B photoreceptor UVR8 on root growth of Arabidopsis
In the present study, wild type (Ler) and its uvr8 mutant were selected to further investigate whether UV-B inhibition of root growth of Arabidopsis was related to the photoreceptor UVR8. When Arabidopsis was cultured in different light environments, the uvr8 mutant showed shorter root length than wild type (Ler) under UV-B, while the two showed no significant difference in white light environment (). The results showed that UVR8 did not act as photoreceptor at least in the inhibition of root growth of Arabidopsis by UV-B, and other photoreceptors were probably involved, while UVR8 was probably a negative regulator in this process. There are more than 20 UVR8-like proteins in Arabidopsis, Citation39 but none of them are involved in UV-B sensitivity.Citation25 However, other structural proteins or other substances are not excluded to act as UV-B photoreceptors. The production of tryptophan photoproducts in animal cells is the primary condition for UV-B response.Citation40 Biever found that UV-B inhibits the hypocotyl growth of xanthated seedlings without the involvement of UVR8. Citation41 DNA as chromophore is sensitive to UV-B during UV-B-induced plant photopoiesis.Citation42 UVR8 was not required to participate in certain UV-B-induced signaling pathways, and there were other UV-B sensing mechanisms in plants.Citation42–Citation45 UVR8 was necessary for the normal development and expansion of leaves, and it regulated the internal replication of leaf cells and stomatal differentiation, but UV-B inhibited the division of leaf epidermal cells without the participation of UVR8 under UV-B environment.Citation44
5. Conclusion
Low-level UV-B inhibits the growth of Arabidopsis root through the inhibition of the division and elongation of root cells. GA restores root length in Arabidopsis under low-level UV-B environment, indicating that the inhibition of root growth by low-level UV-B is achieved by changing the GA3 signal. COP1 is a positive regulator in the process of low-level UV-B inhibiting the root growth of Arabidopsis, while STO is a negative regulator. Low-level UV-B inhibiting the root growth of Arabidopsis is UV-B photoreceptor UVR8 independent, suggesting that receptor involved in UV-B-induced root growth is different from shoot in Arabidopsis.
Abbreviation
Author contributions
Shaoshan Li designed the research; Hongpeng Hu performed the experiment; Guizhen Lyu and Dongbing Li drafted the manuscript; and Guizhen Lyu, Dongbing Li, and Shaoshan Li read and approved the final manuscript.
Conflict of interest
The authors state no conflict of interest.
Acknowledgments
We would like to thank Professor Paul Giller from the University College of Cork, Ireland, for checking the English language of the manuscript.
Additional information
Funding
References
- Verdaguer D, Jansen MAK, Llorens L, Morales LO, Neugart S. UV-A radiation effects on higher plants: exploring the known unknown. Plant Sci. 2017;255:1–8. doi:10.1016/j.plantsci.2016.11.014.
- Ulm R, Nagy F. Signalling and gene regulation in response to ultraviolet light. Curr Opin Plant Biol. 2005;8:477–482. doi:10.1016/j.pbi.2005.07.004.
- Frohnmeyer H, Staiger D. Ultraviolet-B radiation-mediated responses in plants. Balancing Damage and Protection. Plant Physiol. 2003;133:1420–1428.
- Casati P, Walbot V. Crosslinking of ribosomal proteins to RNA in maize ribosomes by UV-B and its effects on translation. Plant Physiol. 2004;136:3319–3332. doi:10.1104/pp.104.047043.
- Jenkins GI. Signal transduction in responses to UV-B radiation. Annu Rev Plant Biol. 2009;60:407–431. doi:10.1146/annurev.arplant.59.032607.092953.
- Heijde M, Ulm R. UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci. 2012;17:230–237. doi:10.1016/j.tplants.2012.01.007.
- Conte M, De Simone S, Simmons SJ, Ballare CL, Stapleton AE. Chromosomal loci important for cotyledon opening under UV-B in Arabidopsis thaliana. BMC Plant Biol. 2010;10:112. doi:10.1186/1471-2229-10-112.
- Kliebenstein DJ, Lim JE, Landry LG. Arabidopsis UVR8 regulates ultraviolet-B signal transduction and tolerance and contains sequence similarity to human regulator of chromatin condensation 1. Plant Physiol. 2002;130:234–243. doi:10.1104/pp.005041.
- Kim BC, Tennessen DJ. UV‐B‐induced photomorphogenesis in Arabidopsis thaliana. Plant J. 1998;15:667–674. doi:10.1046/j.1365-313x.1998.00246.x.
- Vandenbussche F, Tilbrook K, Fierro AC, Marchal K, Poelman D, Der Straeten DV, Ulm R. Photoreceptor-mediated bending towards UV-B in arabidopsis. Mol Plant. 2014;7:1041–1052. doi:10.1093/mp/ssu039.
- Hayes S, Velanis CN, Jenkins GI, Franklin KA. UV-B detected by the UVR8 photoreceptor antagonizes auxin signaling and plant shade avoidance. Proc Natl Acad Sci U S A. 2014;111:11894–11899. doi:10.1073/pnas.1403052111.
- Zhang R, Huang G, Wang L, Zhou Q, Huang X. Effects of elevated ultraviolet-B radiation on root growth and chemical signaling molecules in plants. Ecotoxicol Environ Saf. 2019;171:683–690. doi:10.1016/j.ecoenv.2019.01.035.
- Yokawa K, Kagenishi T, Baluska F. Root photomorphogenesis in laboratory-maintained Arabidopsis seedlings. Trends Plant Sci. 2013;18:117–119. doi:10.1016/j.tplants.2013.01.002.
- Ulm R, Baumann A, Oravecz A, Mate Z, Adam E, Oakeley EJ, Schafer E, Nagy F. Genome-wide analysis of gene expression reveals function of the bZIP transcription factor HY5 in the UV-B response of Arabidopsis. Proc Natl Acad Sci U S A. 2004;101:1397–1402. doi:10.1073/pnas.0308044100.
- Achard P, Gusti A, Cheminant S, Alioua M, Dhondt S, Coppens F, Beemster GTS, Genschik P. Gibberellin signaling controls cell proliferation rate in Arabidopsis. Current Biol. 2009;19:1188–1193. doi:10.1016/j.cub.2009.05.059.
- Schaller GE, Bishopp A, Kieber JJ. The Yin-Yang of hormones: cytokinin and auxin interactions in plant development. Plant Cell. 2015;27:44–63. doi:10.1105/tpc.114.133595.
- Ioio RD, Linhares FS, Scacchi E, Casamitjanamartinez E, Heidstra R, Costantino P, Sabatini S. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Current Biol. 2007;17:678–682. doi:10.1016/j.cub.2007.02.047.
- Riefler M, Novak O, Strnad M, Schmulling T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell. 2006;18:40–54. doi:10.1105/tpc.105.037796.
- Ko D, Kang J, Kiba T, Park J, Kojima M, Do J, Kim KY, Kwon M, Endler A, Song W. Arabidopsis ABCG14 is essential for the root-to-shoot translocation of cytokinin. Proc Natl Acad Sci USA. 2014;111:7150–7155. doi:10.1073/pnas.1321519111.
- Kalbina I, Li S, Kalbin G, Bjorn LO, Strid A. Two separate uv-b radiation wavelength regions control expression of different molecular markers in arabidopsis thaliana. Plant Biol. 2008;35:222-227.
- Kami C, Lorrain S, Hornitschek P, Fankhauser C. Light-regulated plant growth and development. Curr Top Dev Biol. 2010;91:29–66. doi:10.1016/S0070-2153(10)91002-8.
- Rizzini L, Favory J, Cloix C, Faggionato D, Ohara A, Kaiserli E, Baumeister R, Schafer E, Nagy F, Jenkins GI. Perception of UV-B by the Arabidopsis UVR8 protein. Science. 2011;332:103–106. doi:10.1126/science.1200660.
- Brown BA, Cloix C, Jiang GH, Kaiserli E, Herzyk P, Kliebenstein DJ, Jenkins GI. A UV-B-specific signaling component orchestrates plant UV protection. Proc Natl Acad Sci U S A. 2005;102:18225–18230. doi:10.1073/pnas.0507187102.
- Favory J, Stec A, Gruber H, Rizzini L, Oravecz A, Funk M, Albert A, Cloix C, Jenkins GI, Oakeley EJ. Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. Embo J. 2009;28:591–601. doi:10.1038/emboj.2009.4.
- Tilbrook K, Arongaus AB, Binkert M, Heijde M, Yin R, Ulm R. The UVR8 UV-B photoreceptor: perception, signaling and response. Arabidopsis Book. 2013;11. doi:10.1199/tab.0164.
- Yi C, Deng XW. COP1 – from plant photomorphogenesis to mammalian tumorigenesis. Trends Cell Biol. 2005;15:618–625. doi:10.1016/j.tcb.2005.09.007.
- Saijo Y, Sullivan JA, Wang H, Yang J, Shen Y, Rubio V, Ma L, Hoecker U, Deng XW. The COP1–SPA1 interaction defines a critical step in phytochrome A-mediated regulation of HY5 activity. Genes Dev. 2003;17:2642–2647. doi:10.1101/gad.1122903.
- Oravecz A, Baumann A, Mate Z, Brzezinska A, Molinier J, Oakeley EJ, Adam E, Schafer E, Nagy F, Ulm R. CONSTITUTIVELY PHOTOMORPHOGENIC1 is required for the UV-B response in Arabidopsis. Plant Cell. 2006;18:1975–1990. doi:10.1105/tpc.105.040097.
- Jiang L, Wang Y, Li Q, Björn LO, He J, Li S. Arabidopsis STO/BBX24 negatively regulates UV-B signaling by interacting with COP1 and repressing HY5 transcriptional activity. Cell Res. 2012;22:1046–1057. doi:10.1038/cr.2012.34.
- Caldwell MM, Ballaré CL, Bornman JF, Flint SD, Björn LO, Teramura AH, Kulandaivelu G, Tevini M. Terrestrial ecosystems, increased solar ultraviolet radiation and interactions with other climatic change factors. Photochem Photobiol Sci. 2003;2:29–38.
- Heo J-O, Chang KS, Kim IA, Lee M-H, Lee SA, Song S-K, Lee MM, Lim J. Funneling of gibberellin signaling by the GRAS transcription regulator SCARECROW-LIKE 3 in the Arabidopsis root. Proc Nat Aca Sci. 2011;108:2166.
- Dhonukshe P, Tanaka H, Goh T, Ebine K, Mähönen AP, Prasad K, Blilou I, Geldner N, Xu J, Uemura T, et al. Generation of cell polarity in plants links endocytosis, auxin distribution and cell fate decisions. Nature. 2008;456:962. doi:10.1038/nature07409.
- Cary AJ, Liu W, Howell SH. Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyl elongation in Arabidopsis thaliana seedlings. Plant Physiol. 1995;107:1075–1082. doi:10.1104/pp.107.4.1075.
- Werner T, Nehnevajova E, Kollmer I, Novak O, Strnad M, Kramer U, Schmulling T. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell. 2010;22:3905–3920. doi:10.1105/tpc.109.072694.
- Dyachok J, Zhu L, Liao F, He J, Huq E, Blancaflor EB. SCAR mediates light-induced root elongation in arabidopsis through photoreceptors and proteasomes. Plant Cell. 2011;23:3610–3626. doi:10.1105/tpc.111.088823.
- Sassi M, Lu Y, Zhang Y, Wang J, Dhonukshe P, Blilou I, Dai M, Li J, Gong X, Jaillais Y. COP1 mediates the coordination of root and shoot growth by light through modulation of PIN1- and PIN2-dependent auxin transport in Arabidopsis. Development. 2012;139:3402–3412. doi:10.1242/dev.078212.
- Indorf M, Cordero J, Neuhaus G, Rodriguezfranco M. Salt tolerance (STO), a stress-related protein, has a major role in light signalling. Plant J. 2007;51:563–574. doi:10.1111/j.1365-313X.2007.03162.x.
- Kumagai T, Ito S, Nakamichi N, Niwa Y, Murakami M, Yamashino T, Mizuno T. The common function of a novel subfamily of B-Box Zinc finger proteins with reference to circadian-associated events in Arabidopsis thaliana. Biosci Biotechnol Biochem. 2008;72:1539–1549. doi:10.1271/bbb.80041.
- Kuhn K, Carrie C, Giraud E, Wang Y, Meyer EH, Narsai R, Des Francssmall CC, Zhang B, Murcha MW, Whelan J. The RCC1 family protein RUG3 is required for splicing of nad2 and complex I biogenesis in mitochondria of Arabidopsis thaliana. Plant J. 2011;67:1067–1080. doi:10.1111/j.1365-313X.2011.04658.x.
- Fritsche E, Schafer C, Calles C, Bernsmann T, Bernshausen T, Wurm M, Hubenthal U, Cline JE, Hajimiragha H, Schroeder P. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc Natl Acad Sci U S A. 2007;104:8851–8856. doi:10.1073/pnas.0701764104.
- Biever, JJ, Brinkman, D, Gardner, G. UV-B inhibition of hypocotyl growth in etiolated Arabidopsis thaliana seedlings is a consequence of cell cycle arrest initiated by photodimer accumulation. J Exp Bot. 2014;65:2949–2961. doi:10.1093/jxb/eru035.
- Shinkle JR, Atkins AK, Humphrey EE, Rodgers CW, Wheeler SL, Barnes PW. Growth and morphological responses to different UV wavebands in cucumber (Cucumis sativum) and other dicotyledonous seedlings. Physiol Plant. 2004;120:240–248. doi:10.1111/j.0031-9317.2004.0237.x.
- Besteiro MAG, Bartels S, Albert A, Ulm R. Arabidopsis MAP kinase phosphatase 1 and its target MAP kinases 3 and 6 antagonistically determine UV-B stress tolerance, independent of the UVR8 photoreceptor pathway. Plant J. 2011;68:727–737. doi:10.1111/j.1365-313X.2011.04725.x.
- Wargent JJ, Gegas VC, Jenkins GI, Doonan JH, Paul ND. UVR8 in Arabidopsis thaliana regulates multiple aspects of cellular differentiation during leaf development in response to ultraviolet B radiation. New Phytol. 2009;183:315–326. doi:10.1111/j.1469-8137.2009.02855.x.
- Brown BA, Jenkins GI. UV-B signaling pathways with different fluence-rate response profiles are distinguished in mature Arabidopsis leaf tissue by requirement for UVR8, HY5, and HYH. Plant Physiol. 2007;146:576–588. doi:10.1104/pp.107.108456.
