ABSTRACT
As functional proteins dehydrins are found in many maturing seeds and vegetable tissue under adverse environmental conditions. However, the regulation of dehydrin expression remains unclear.
To explore regulatory mechanisms of wheat dehydrin WZY2 expression under abiotic stresses, we constructed a cDNA library from PEG- and cold-treated wheat seedlings and performed yeast one-hybrid assay and yeast two-hybrid assay to identify the upstream transcription factors and protein interacting with dehydrin WZY2 gene. Yeast one-hybrid assay illustrated that bHLH49-like (GenBank NO. XM_020296294), zinc finger A20 and AN1 domain-containing stress-associated protein 6-like (GenBank NO. XM_020341647), and bHLH47-like (GenBank NO. XM_020313116) proteins can bind and interact with the promoter of the WZY2, these sequences are Aegilops tauschii transcription factors, the diploid progenitor of the D genome of hexaploid wheat (Triticum aestivum, genomes AABBDD). Real-time PCR analyzes unraveled the stress responsive expression of XM_020296294, XM_020341647, and XM_020313116 in wheat. XM_020296294 and XM_020341647 showed a similar expression patterns with WZY2. Yeast two-hybrid assay indicated that PP2C (GenBank NO. XM_020293398) protein can interact with WZY2. These results provided evidences that WZY2 could be positively regulated by XM_020296294 and XM_020341647 transcription factors, and WZY2 may also play an important role in the ABA signaling pathway through interaction with PP2C to regulate stress-responsive genes expression in wheat. The obtained results contribute for provide a better understanding of the regulatory mechanism of dehydrin expression under abiotic stresses in wheat.
In the course of evolution plants have developed complex regulatory mechanisms to respond and sustain abiotic stresses. The regulation mechanism of abiotic stress in plants mainly depends on two groups of proteins: one containing mainly proteins functioning in direct abiotic tolerance [e.g. late embryogenesis abundant (LEA) proteins], and the other consisting of regulators for intracellular signaling and stress‐inducible gene expression (e.g. protein kinases such as MAP kinases, phosphatases, phospholipid metabolic enzymes, and various types of transcription factors).Citation1 The late embryogenesis abundant (LEA) protein are an important group of functional proteins to reduce cell damage and protect cells under abiotic stress conditions.Citation1,Citation2 Dehydrins (DHNs), also known as LEA II (late embryogenesis abundant) proteins, accumulate during late seed developmental stages and in vegetative tissues subjected to dehydration, salt, and low temperature stresses.Citation3 Dehydrins have been confirmed to play a fundamental role in plant response to various abiotic and biotic stresses.Citation4–Citation6 However, there is relatively little discussion about the upstream regulatory mechanisms of the expression of dehydrins under adverse environmental conditions.
In our previous study, we cloned a YSK2-type dehydrin gene WZY2 (GenBank NO. EU395844) and its promoter from wheat.Citation7,Citation8 Real-time PCR analysis showed that the expression of WZY2 could be induced by low temperature, and ABA treatments. Histochemical analysis of GUS expression also demonstrated that WZY2 promoter activity could be up-regulated by low temperature and ABA treatments.Citation7 Compared to the wild-type, transgenic RNAi wheat line showed lower relative water content, lower oxidative-related enzyme activities and higher malondialdehyde (MDA) content under drought stress.Citation9 Overexpression of the WZY2 in Arabidopsis also revealed a significant increase drought stress tolerance.Citation9 Our previous results demonstrated the important function of WZY2 for the achievement of abiotic stresses tolerance in wheat.
In order to understand regulatory mechanisms of dehydrin WZY2 under abiotic stresses, we constructed a cDNA library from 20% PEG600- and cold (4°C)-treated wheat seedlings and performed yeast one-hybrid (Y1H) assay with the WZY2 promoter (Pwzy2) as bait to identify putative transcription factors regulating dehydrin WZY2. By sequencing the positive clones screened by yeast one-hybrid assay, we isolated 351 genes which putatively interacting with the WZY2 promoter. Among them, we detected 14 transcription factors (). To further distinguish between genuine positive from false positive interactions, the plasmids containing the transcription factor genes were extracted, and respectively retransformed into Y1H Gold yeast strains and Y1H Gold (Pwzy2) bait yeast strains integrating the WZY2 promoter (Pwzy2) into the Y1HGold yeast genome. The yeast one-hybrid results showed that Y1H Gold yeast cells containing pGADT7-XM_020296294/XM_020341647/XM_020313116 could not grow on SD/-Leu/AbA200 selective medium. However, Y1H Gold (Pwzy2) bait yeast strains containing pGADT7-XM_020296294/XM_020341647/XM_020313116 were able to grow on SD/-Leu and SD/-Leu/AbA200 selective medium (). None of the Y1H Gold or Y1H Gold (Pwzy2) transformed with the remaining 11 transcription factors could grow on SD/-Leu/AbA200 selective medium. These results indicated XM_020296294, XM_020341647, and XM_020313116 were probable upstream regulators of WZY2.
Table 1. Putative transcription factors interacting with dehydrin WZY2 promoter.
Figure 1. Identification upstream transcription factors and an interacting protein of dehydrin WZY2 gene. (a) The interaction of XM_020296294, XM_020341647, and XM_020313116 with the promoter of WZY2 (Pwzy2) in Y1H Gold yeast cells. (b) Relative expression level of XM_020296294, XM_020341647, XM_020313116, and WZY2 under different abiotic stress. Wheat seedlings were treated with 20% PEG6000, cold (4℃) and 100 μM ABA. Total RNA was isolated from leaves at the indicated times after the treatments. The expression levels were relative to the wheat endogenous actin gene (Accession No. AB181991) under abiotic stress treatments. (c) The interaction of PP2C with WZY2 in Y2H Gold yeast cells.
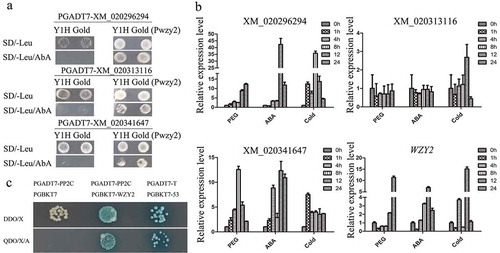
To further explore the involvement of XM_020296294, XM_020341647, and XM_020313116 transcription factors in regulation of dehydrin WZY2 expression, we evaluated the transcript abundance of XM_020296294, XM_020341647, XM_020313116, and WZY2 in leaves of wheat seedlings subjected to 20% PEG6000, cold (4°C) and 100 μM ABA treatments, at different time points (0, 1, 4, 8, 12 and 24 h) (), performed as described previously.Citation10,Citation11 Real-time PCR results indicated that XM_020296294, XM_020341647, and WZY2 share similar expression patterns manifested with an early accumulation of the tested transcripts with subsequent diminishing levels as the PEG, cold and ABA treatment progressed. The XM_020313116 expression remained unaffected by the imposed stresses. These results indicated that XM_020296294, XM_020341647 were potential positive regulators of WZY2 expression.
In order to explore further the mechanism of dehydrin WZY2 gene response to abiotic stresses, we also screened for candidate proteins interacting with dehydrin WZY2 by yeast two-hybrid system. By sequencing the positive clones screened by yeast two-hybrid assay, we isolated a protein phosphatase 2C (GenBank NO. XM_020293398) which putatively interacting with the WZY2 (). The protein phosphatase 2C (PP2C) is a key protein in the ABA signaling pathway. In the absence of ABA, PP2C cannot bind to PYR/PYL/RCAR, but interacts with SnRK2 kinase to inhibit its activity. Under elevated levels of ABA, PP2C binds to PYR/PYL/RCAR, thus blocking SnRK2 inhibition, which enables the phosphorylation of downstream targets such as transcription factors.Citation12 In summary, XM_020296294 and XM_020341647 activate WZY2 expression by binding to the activator region of the WZY2 promoter. WZY2 may also play an important role in the ABA signaling pathway through interaction with PP2C to regulate stress-responsive gene expression in wheat.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download MS Word (19.4 KB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Hirayama T, Shinozaki K. Research on plant abiotic stress responses in the post-genome era: past, present and future. Plant J. 2010;61:1–3. doi:10.1111/j.1365-313X.2010.04124.x.
- Debnath M, Pandey M, Bisen PS. An omics approach to understand the plant abiotic stress. OMICS. 2011;15:739–762. doi:10.1089/omi.2010.0146.
- Koag MC, Wilkens S, Fenton RD, Resnik J, Vo E, Close TJ. The K-segment of maize DHN1 mediates binding to anionic phospholipid vesicles and concomitant structural changes. Plant Physiol. 2009;150:1503–1514. doi:10.1104/pp.109.136697.
- Mota A, Oliveira TN, Vinson CC, Williams T, Costa M, Araujo A, Danchin EGJ, Grossi-de-Sá MF, Guimaraes PM, Brasileiro ACM. Contrasting effects of wild arachis dehydrin under abiotic and biotic stresses. Front Plant Sci. 2019;10:497. doi:10.3389/fpls.2019.00497.
- Vaseva II, Anders I, Yuperlieva-Mateeva B, Nenkova R, Kostadinova A, Feller U. Dehydrin expression as a potential diagnostic tool for cold stress in white clover. Plant Physiol Biochem. 2014;78:43–48. doi:10.1016/j.plaphy.2014.02.014.
- Hanin M, Brini F, Ebel C, Toda Y, Takeda S, Masmoudi K. Plant dehydrins and stress tolerance: versatile proteins for complex mechanisms. Plant Signal Behav. 2011;6:1503–1509. doi:10.4161/psb.6.10.17088.
- Zhu W, Zhang L, Lv H, Zhang H, Zhang D, Wang X, Chen J. The dehydrin wzy2 promoter from wheat defines its contribution to stress tolerance. Funct Integr Genomic. 2014;14:111–125. doi:10.1007/s10142-013-0354-z.
- Yang W, Zhang L, Lv H, Li H, Zhang Y, Xu Y, Yu J. The K-segments of wheat dehydrin WZY2 are essential for its protective functions under temperature stress. Front Plant Sci. 2015; 6: 406.
- Yu Z, Wang X, Mu X, Zhang L. RNAi mediated silencing of dehydrin gene WZY2 confers osmotic stress intolerance in transgenic wheat. FPB. 2019;46:877–884. doi:10.1071/FP19068.
- Zhu W, Zhang D, Lu X, Zhang L, Yu Z, Lv H, Zhang H. Characterisation of an SKn-type dehydrin promoter from wheat and its responsiveness to various abiotic and biotic stresses. Plant Mol Biol Rep. 2014;32:664–678. doi:10.1007/s11105-013-0681-1.
- Liu H, Xing M, Yang W, Mu X, Wang X, Lu F, Wang Y, Zhang L. Genome-wide identification of and functional insights into the late embryogenesis abundant (LEA) gene family in bread wheat (Triticum aestivum). Sci Rep-UK. 2019;9:13375. doi:10.1038/s41598-019-49759-w.
- Sreenivasulu N, Harshavardhan VT, Govind G, Seiler C, Kohli A. Contrapuntal role of ABA: does it mediate stress tolerance or plant growth retardation under long-term drought stress?. GENE. 2012;506:265–273. doi:10.1016/j.gene.2012.06.076.
