?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Stomatal behavior under global climate change is a central topic of plant ecophysiological research. Vapor pressure deficit (VPD) and phytohormones can affect stomata of leaves which can affect gas exchange characteristics of plant. The role of VPD in regulating leaf gas exchange of three tree species was investigated in Jinan, China. Experiments were performed in June, August, and October. Levels of three phytohormones (GA3, IAA, ABA) in the leaves of the three trees were determined by high-performance liquid chromatography in three seasons. The responses of stomatal conductance (gs) to an increasing VPD in the leaves of the three trees had peak curves under different seasons, which differed from the prevailing response pattern of gs to VPD in most literature. The peak curve could be fitted with a Log-Normal Model (R2 = 0.838–0.995). The VPD/RH values of the corresponding maximum of gs (gs-max-VPD/RH) could be calculated by fitted models. The gs-max-RH could be affected by environmental conditions, because of positive correlation between gs-max-RH and the mean monthly temperature in 2010 (R2 > 0.81). Two typical stomatal models (the Leuning model and the optimal stomatal behavior model) were used to estimate gs values, but they poorly predicted gs in the three trees. The concentration of ABA was positively correlated to sensitivity in response of stomatal conductance to VPD in the leaves of the tree species during the different seasons.
1. Introduction
Stomata are the major pathway for acquiring carbon and limiting water loss between vegetation and the atmosphere. Stomatal behavior under global climate change is a central topic of plant ecophysiological research because it affects plant growth, vegetation distribution and ecosystem function. Global climate change significantly affects plant productivity and their water transport and use patterns, which would be reflected in the water use efficiency (WUE) of individual plants, communities, and ecosystems, and ultimately, in the vegetation distribution pattern, species composition, and ecosystem structure. Therefore, it is important to understand stomatal movement because it is a factor in changing the planet and in modeling the climate and weather of the current and future Earth.Citation1-Citation4 To study the WUE of plants would be helpful in understanding and forecasting the responses of terrestrial vegetation to global climate change and to the adoption of adaptive strategies. In addition to the environmental factors which affect the stomatal regulation, including light, temperature, relative humidity, etc., phytohormones also participate in the stomatal regulation, including ABA, auxin, cytokinin, ethylene, gibberellin, etc.Citation5-Citation7
The prevailing pattern regarding the relationship between gs and atmospheric water content is that increasing vapor pressure deficit (VPD) leads to reduction of gs.Citation8-Citation11 Stomatal conductance is closely linked to atmospheric water content.Citation12 The Ball-Woodrow-Berry (BWB) model employs a linear relationship between the stomatal conductance (gs) and relative humidity (RH).Citation8 However, the models based on vapor pressure deficit generally perform better than those based on relative humidity.Citation13 VPD is a more appropriate variable in describing stomatal responses to air humidity;Citation14 therefore, relative humidity is replaced by vapor pressure deficit in the stomatal response models after Leuning’ model publication.Citation15 The main stomatal models until now could be divided into two types. One type is the empirical model which is widely used because of readily estimated parameters from measured data and simple implementation at different scales. The typical empirical models were developed by Jarvis,Citation14 Ball et al.,Citation8 LeuningCitation15 et al. The other type of stomatal model is based on the theory of optimal stomatal behavior.Citation16 Although abundant research has been carried out about stomatal response and many models have been established, the physiological mechanism controlling the response of stomata to VPD (or RH) is inadequately understood.Citation8,Citation13-Citation15
Some of the present stomatal conductance models have shown poor predictions compared with measured data. For example, evaluation of gs by Jarvis’ model was better than the BWB model with Aneurolepidium chinense, Citation17 but both stomata models poorly predicted values of gs under high RH conditions.Citation13 Liu et al.Citation18 suggested that the Leuning model may not be appropriate for measured data analysis and ecosystem simulation applications in arid and semiarid zones by comparing the predicted with measured data of three major species in a semi-arid site. In addition, many studies have shown different patterns of the response of gs to VPD. Vitis Pseudoreticulata indicates a maximal gs at RH 70% with increasing RH, which showed a stress to stomatal regulation at high RH.Citation19 Three distinct phases were used to describe the pattern of gs to VPD response in Pseudotsuga menziesiiCitation12 with gs first increasing then decreasing when VPD increased. The leaves of Pseudotsuga menziesii showed a maximal gs at intermediate VPD. Soni et al.Citation20 also have reported similar relationships between stomatal conductance and VPD in Selaginella bryopteris. High RH reduces the efficacy of transpiration.Citation21-Citation23 All of the above-mentioned reports concerning the response of gs to VPD show a different response pattern of gs to VPD from the prevailing opinion. However, little scholars pay attention to explore or analyze the environmental or plant characteristics and the possible causes of this phenomenon.
The response of stomata to environmental and physiological factors is complex. Phytohormones are important factors that affect stomatal regulation. Increased levels of abscisic acid (ABA) while a plant is under stress promote stomatal closure and/or inhibit stomata opening in order to avoid excessive water loss.Citation24-Citation29 The response of gs to VPD is correlated to the level of ABA.Citation30 The other phytohormones, including indole-3-acetic acid (IAA) and Gibberellins (GAs), are known to antagonize the effects of ABA on stomatal behavior.Citation31,Citation32 High ratios of GA3/ABA and IAA/ABA been have shown to maintain stomata open in order to keep growing under high temperature and drought conditions.Citation33 Larger ratios of IAA/ABA could lead to better growth of plants,Citation34 and also GA3/ABA affected the growth rate of plants.Citation35,Citation36
It is important and urgent to study the stomatal behavior, and understand the characteristics of stomatal and physiological factors’ response to global climate change. Therefore, in this study, the response patterns of stomatal conductance to VPD in three temperate trees under different seasons were characterized. The characteristics of these response patterns are discussed. We also investigated whether the phytohormones in the leaves of the three trees had an influence on the relationship between gs and VPD. In addition, the two stomatal models (Leuning model and the optimal stomatal model) were simulated and we compared the predicted and measured stomatal conductance values in the leaves of the three trees in different seasons.
2. Materials and methods
2.1. Sites description
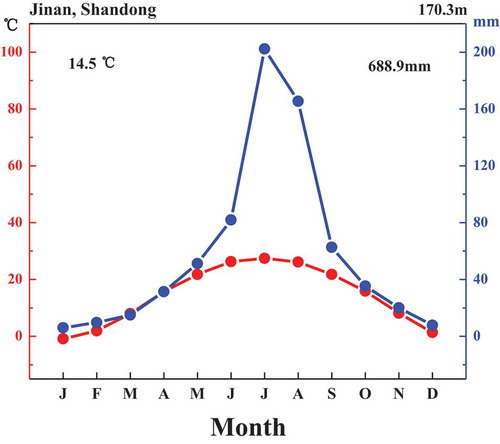
The study site is located in Jinan (36°35′36”-36°40′04” N, 116°54′29”-117°02′01” E), Shandong Province, China. Jinan is located in a typical warm-temperate humid/semi-humid climate which is characterized by a long summer with mean annual precipitation of 688.9 mm. The regional dominant vegetation belongs to needle-broadleaved mixed forest. The climate diagram is shown in . The climatic data were collected from the China Meteorological Administration (http://www.cma.gov.cn/), and show average values from 1951 to 2010.Citation37
2.2. Plant material
Fraxinus chinensis Roxb., Populus alba L. var. pyramidalis Bge. and Populus tomentosa Carr. are widely distributed in China. Fraxinus chinensis Roxb. grow rapidly and are distributed widely in both north and south of China. Populus alba L. var. pyramidalis Bge. has the natural distribution area in the north of China with drought and salinity tolerance. Populus tomentosa Carr. is distributed in the central and northern parts of China and its optimum tree specie for shelter forest. All three tree species are also excellent gree tree species. Therefore, they were chosen to measure the response characteristics of stomatal conductance to VPD in different seasons as the typical tree species.
P. alba var. and P. tomentosa were 1-year-old seedlings. Cuttings (20–25 cm) of the same clone were collected from Urumqi (Xinjiang Uygur Autonomous Region) for P. alba var., and from Jinan (Shandong Province) for P. tomentosa. The cuttings were planted in plastic pots outside at the end of March 2010. The plants were well watered every day and supplemented with Hoagland nutrient solution once a week. The F. chinensis were adult trees.
Measurements were made on the leaves of the plants. The regimes of the experimental plants and the leaf chamber temperature (Tb) during measurements are shown in .Citation37
Table 1. Features of the experimental plants and leaf chamber temperature (Tb) during measurements.
2.3. Gas exchange measurements
Measurements of leaf stomatal response to air humidity in the three trees were carried out on sunny days in June (early summer), August (midsummer) and October (late autumn), 2010. The 3–7 individuals for each species of the three trees were selected to measure leaf stomatal conductance response, with fully developed and sun-exposed leaves in adult trees and the seventh/eighth leaves from the top in seedlings, between 8:00 and 12:00 am (to avoid midday depression in photosynthesis) in Jinan,Citation38-Citation40 using a portable photosynthesis system LI-COR LI-6400 equipped with a red/blue LED source (LI-COR Inc., Lincoln, NE, USA). Photosynthetic photon flux density (PPFD) at 8:00 am (PPFD >200 μmol/(m2·s)) could exceed the light compensation point for most tree species in Jinan.Citation41-Citation44 Prior to leaf gas exchange measurements, cuvette PPFD was adjusted to 1500 μmol/(m2·s) as light saturation and constant leaf chamber temperature (, the third column) with ambient CO2 content in order to determine the stomatal conductance response to vapor pressure deficit. The vapor pressure deficit was controlled with the LI-COR 6400 humidity control function.
2.4. Stomatal response models
Two typical stomatal models were used to compare the estimated gs and measured gs data. One is the Leuning modelCitation15 as one of the empirical models; the other is the optimal stomatal model which was modified by Medlyn et al.Citation45,Citation46
The Leuning model reads
where gs is the stomatal conductance; An is the net photosynthetic rate; D and Cs are vapor pressure deficit and the CO2 concentration at the leaf surface, respectively; g0 is the conductance as An→0 when leaf irradiance→0; and D0 and a are empirical coefficients.
The optimal stomatal model reads
where gs is the stomatal conductance; An is the net photosynthetic rate; D and Cs are vapor pressure deficit and the CO2 concentration at the leaf surface, respectively; g0 is the conductance as An→0 when leaf irradiance→0; and gl and a are empirical coefficients.
2.5. Phytohormone measurements
Measurement of the leaf phytohormones in this study was done by high-performance liquid chromatography (HPLC). Plant leaf samples were collected and powdered with liquid nitrogen, and extracted with cold methyl alcohol at 4 °C. After centrifugation (8000 rpm, 4 °C, 10 min) and complete removal of the methyl alcohol with a rotating evaporator, the dried samples were dissolved in phosphate buffer solution (pH 8.0) and chloroform. Further clean-up was achieved by using chloroform and cross-linked polyvinylpyrrolidone (PVPP) to remove pigment and phenols, respectively. Final clean-up of the extract was done by extracting with ethyl acetate at pH 2.8 (adjusted with formic acid). Finally, the extracts were centrifuged, evaporated and then the dried samples were stored in 1.5 ml of mobile phase solution (methanol/H2O, 40/60, 0.5% acetic acid) until HPLC analysis was performed. The concrete step about extraction and purification of the leaves followed the procedure described by Fu et al.Citation47 Detections limits and recoveries of standards of GA3, IAA, and ABA are shown in .Citation37
Table 2. Detection limits and recoveries of standards of GA3, IAA, and ABA.
Phytohormones were monitored on a diode array detector (SPD-M10Avp) with detection wavelength at λ 254 nm using HPLC (Shimadzu Lc-10ADvp, Japan). The samples were separated on a C18 reversed-phase column using the above mobile phase solution, the column temperature was to keep at 38 °C. All reagents were HPLC-grade. The GA3, IAA and ABA standards were purchased from Sigma (St. Louis, USA).
2.6. Statistical analysis
Significant differences among multiple means in the concentration of phytohormones were tested using a least significant difference test (SPSS v. 16.0). The response curves were fitted using non-linear regression method by SigmaPlot (v. 10.0). The differences between our measured data and simulated data based on the two models were, respectively, tested with F-test (SPSS) and Root Mean Square Error (RMSE, σ).
The sensitivity of gs to VPD in the published reports was described by .Citation48,Citation49 The change trend of gs to VPD was assumed to decrease monotonously in those researches. However, the result in this paper showed that the change trend of gs to VPD was one top line in three tree species in different seasons. So the test of the sensitivity of gs to VPD in the three tree species in the three different seasons was carried out using Root Mean Square Error of stomatal conductance in a certain range of VPD (Equation 3). RMSE could be a more appropriate way to reflect the sensitivity of gs to VPD.
RMSE shows the variation from the average.Citation48 A high σ indicates that the data points tend to be very far from the mean.
where gs is the measured value; is the value of y = x in expressing the gs model prediction, and
is the average value of measured data in expressing the sensitivity of gs to VPD; n is the number of the measured samples.
3. Results
The experiment was conducted in June (early summer), August (midsummer) and October (late autumn), 2010 in the central campus of Shandong University in Jinan. The net photosynthetic rate (An), transpiration rate (E) and gs were determined with a gradient of increasing VPD at constant PPFD and Tb applied to the measured leaf in the cuvette (). shows the responses of gs, An, E and WUE to VPD in the leaves of F. chinensis, P. alba. var. and P. tomentosa in June, August, and October in Jinan.Citation37 shows the concentrations of ABA, GA3, IAA and the ratios of GA3/ABA and IAA/ABA in different seasons.Citation37
Table 3. The concentration of phytohormone (GA3, IAA, ABA) and the ratios of GA3/ABA and IAA/ABA in three tree species in Jinan.
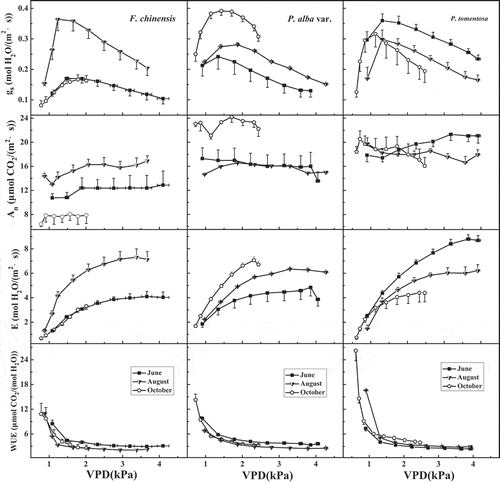
Figure 2. The responses of stomatal conductance (gs), net photosynthesis rate (An), transpiration rate (E) and water use efficiency (WUE) to vapor pressure deficit (VPD) in F. chinensis, P. alba var. and P. tomentosa in Jinan, China. Error bars represent standard errors of gs, An and E with 3–7 replicates measurements.
3.1. The response of gas exchange to VPD
shows the variations of stomatal conductance, photosynthetic rates, transpiration rates, and water use efficiency in the three trees along a gradient of vapor pressure deficit. There is a particular stomatal response pattern to VPD in our results, which shows that gs is low under low VPD, and gs increases with increasing VPD following by a peak at intermediate VPD, and then gs declines steadily with further increases in VPD. There is a maximal gs (gs-max) with increasing VPD. This stomatal response pattern to high VPD shows a similar trend with most stomatal response patterns to VPD at high VPD in the literature,Citation9-Citation11 which also show that gs decreased with further increasing VPD. Photosynthetic rates show a smoother change with increasing VPD in the three trees, but a significant increase in E with increasing VPD.
An, E and gs of F. chinensis were highest in August; An, E, and gs of P. alba. var. were highest in October.
3.2. The response of WUE to VPD
Water use efficiency (WUE) reflects the relationship between photosynthetic production and water consumption.Citation50 The instantaneous WUE is calculated as the ratio of An to E which could be affected by light, temperature, relative humidity, etc. The three trees show the same pattern of WUE to VPD, that is WUE decreased gradually with increasing VPD. Under low VPD, WUE decreased sharply due to the steep increase in E (). However, WUE decreased slowly and smoothly with further increased in VPD. Variations in WUE along a gradient of increasing VPD are shown in .
3.3. Models test
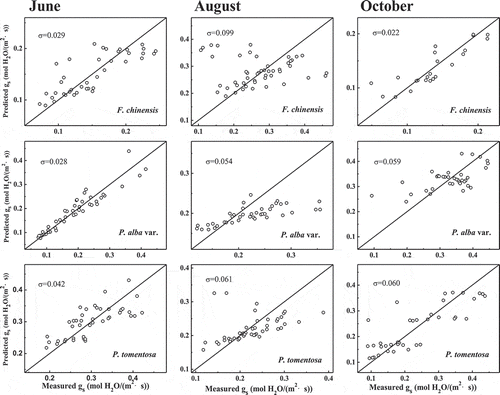
and show a comparison of the measured data with the simulated stomatal conductance provided by the two models.Citation37 There are obvious differences between the measured data and the predicted data.
Figure 3. Comparison of stomatal conductance predicted by the Leuning model and measured data for the three tree species in Jinan, China. The diagonal line is the 1:1 relationship between predicted data and measured data. The σ value is the difference between the 1:1 line and measured data (Root Mean Square Error, RMSE).
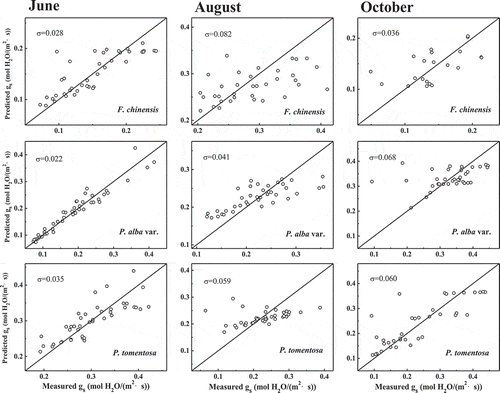
Figure 4. Comparison of stomatal conductance predicted by the optimal stomatal model and measured data for the three tree species in Jinan, China. The diagonal line is the 1:1 relationship between predicted data and measured data. The σ value is the difference between the 1:1 line and measured data (Root Mean Square Error, RMSE).
3.4. Phytohormones in the leaves of the three trees
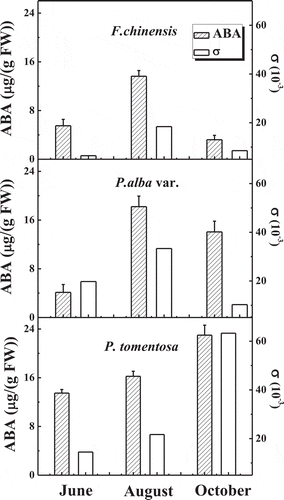
GA3, IAA and ABA concentration in the leaves of the three tree species in Jinan are shown in .Citation37 The measurement results showed that the ABA concentrations in the leaves of the three trees in June were lower than in August. The highest GA3/ABA and IAA/ABA in the leaves were found in all three trees in June.
The concentration of GA3 in the leaves decreased with season in F.chinensis, especially GA3 in late autumn (). The concentration of IAA and ABA changed more moderately than GA3 in the three trees in the different seasons. GA3 concentration in the leaves of F. chinensis decreased sharply, whereas it increased in the leaves of P. alba var. and P. tomentosa in October compared with that in August.
4. Discussion
4.1. Stomatal response to vapor pressure deficit
Our results showed that the stomatal conductance increased with the increasing VPD under low VPD conditions, and reached a peak value under a certain VPD condition; then, the stomatal conductance gradually decreased following a further increasing VPD. This changing pattern of gs to VPD was of a feature of peak curve. Many researchers have shown that the stomatal aperture decreases at high humidity in many tree species.Citation51-Citation54 The stomatal conductance showed a maximal value (gs-max) in a certain range of VPD along with increasing VPD in the three trees, which was similar to the published results by Woodruff et al. in Douglas-fir.Citation12 The response pattern of gs to VPD in this study did not agree with the prevailing stomatal response pattern to VPD.Citation8,Citation9,Citation11 A Log-Normal Model was selected to fit the measured data using a nonlinear regression method (SigmaPlot, V. 10.0). Then, we tested the fitting models, and the results showed high correlation coefficients and goodness of fit (gs = a·exp (−0.5(ln(D/c)/b)2), D = VPD, R2 = 0.845–0.996). The gs-max and VPD/RH corresponding to gs-max (gs-max-VPD/RH) were calculated based on this model ().Citation37 Environmental factors significantly affect the leaves’ performance (like stomatal length, width, and area).Citation55-Citation61 Arve et al. found that the regulation of somatal movement and the response of gs to environmental factors mainly depended on the developing period of the leaves,Citation56,Citation58 which was consistent with our results. Therefore, the gs-max-VPD of leaves from each tree species showed little variation among the different seasons during the experimental period.
Table 4. Nonlinear simulation formula of gs to VPD and gs-max corresponding to VPD and RH.
VPD was calculated from RH and temperature.Citation62 Mean monthly temperature and RH were different in the three seasons. Therefore, the gs-max-RH of the leaves among the seasons was different. The gs-max-RH is about 60–70% in June at about 25.8°C, 57–65% in August at about 25.1°C, and 12–57% in October at about 15.3°C. The trends of gs-max-RH in the three trees in the different seasons and the mean monthly temperature (°C) in different seasons in 2010 were the same (R2 > 0.81) (, the sixth and eighth columns), which shows that gs-max-RH is related to the climatic conditions. shows the fitting formulas, the related correlation coefficients of fitting model, the gs-max-VPD/RH and the mean monthly VPD/RH in 2010.
Therefore, the present data show that an optimal gs occurs at optimal VPD. When VPD is higher or lower than the optimal VPD, gs decreases. The optimal VPD could be affected by the environmental conditions. The pattern in response of gs to VPD could be described with a Log-Normal Model. All three tree species reached the maximum stomatal conductance in a certain range of VPD in the different seasons.
4.2. Comparison of two stomatal models predicting gs with measured gs
Models of stomatal conductance play an important scientific role in summarizing commonly observed trends in stomatal behavior, and advancing our understanding of ecosystem responses to global change. They are widely used for predicting climate impacts on biospheric carbon cycles, hydrological cycles, and nutrient cycles. The prevailing pattern regarding the relationship between gs and atmospheric water content is that increasing VPD leads to the reduction of gs.Citation8–Citation11 Two types of stomatal conductance models are widely used to simulate the gas exchange of leaves.Citation13,Citation17,Citation18 Two typical stomatal models (the Leuning model and the optimal stomatal model) were used to compare the predicted gs and measured gs data.Citation14,Citation45,Citation46 The data of gs under increasing VPD are simulated by the Leuning model and the optimal stomatal model. Both models performed poorly in predicting gs in the three trees ( and ) in different seasons in Jinan. There are obvious differences (P < .05, F-test) in the measured data and the predicted data of the three tree species in August and P. alba var. in October with those two models. The difference between the 1:1 line and the measured data (σ, RMSE) was bigger in the three tree species in August and P. alba var. in October with those two models than the others ( and ). These results show that the present data on the response of stomatal conductance to VPD cannot be explained well with either of the two stomatal models.
4.3. Patterns of WUE in three tree species
The net photosynthesis rate did not show a significant variation with increasing VPD even at high VPD in the three tree species in Jinan. Assimilation continued undiminished in spite of the declined gs at low and high VPD, which demonstrated that An was not sensitive to VPD. However, net photosynthetic rate (An) in the three trees varied strongly among the different species. In addition to the plant species, environment factors (like temperature and precipitation) could lead to the different assimilation performances by the trees.Citation20 Transpiration rates (E) in the three trees showed a steady increase, and increased steeply at the lower range of VPD. These findings were similar to those of Soni et al. in S.bryopteris.Citation20
However, An of F.chinensis in October decreased sharply (, the second row), and the same result was found in gs-max-RH (12.31%) (, the fourth row). IAA, CTK (cytokinin) and GAs could inhibit leaf senescence, GA3 could promote the growth of plant stem and leaf, and the other phytohormones like ABA and ETH (ethylene) could promote leaf senescence.Citation63-Citation67 Growing periods in trees and the environmental conditions could affect the level of phytohormones in the leaves.Citation47 GA3 concentration in the leaves of F. chinensis decreased sharply, which was associated with the senescence in plant leaves.Citation32,Citation66,Citation68 Leaf function was limited by senescence of the leaves of F.chinensis, and An of F.chinensis reduced significantly in October. Therefore, the optimal RH of F.chinensis was just 12.3% in October which showed the poor adaption of the leaf stomata to RH in autumn.
The different response patterns of An and E to VPD lead to a sharp reduction in WUE at low VPD, and then a smooth decrease in WUE was observed. The last row of demonstrates that the WUE was significantly higher at lower ranges of VPD than at higher ranges of VPD for all three species of trees.
4.4. Sensitivity of gs to VPD variation and the concentration of phytohormones
Phytohormones are of vital importance for regulating the stomatal aperture, which is crucial for minimizing water loss and maximizing CO2 exchange. In particular, ABA plays a major role in regulating stomatal function in adaptation to various environmental stresses, such as drought, cold, air pollutants, etc.Citation24-Citation27 Inadequate stomatal closure at high vapor pressure deficit may be mediated by abscisic acid.Citation30 In addition, other phytohormones also contribute to stomatal aperture regulation, such as auxin, cytokinin, ethylene and salicylic acid.Citation31,Citation33,Citation34 Gibberellins and IAA are major phytohormones involved in various aspects of plant growth and development, and they are also known to antagonize the effects of ABA on stomatal movement.Citation31,Citation32
Stomata of different species vary in their sensitivity in response of gs to VPD. The different levels of ABA observed in the different seasons, affect the sensitivity in response of gs to VPD. The response of gs to VPD was very sensitive to the level of ABA because ABA could lead to an obvious inhibition of gs in leaves.Citation69 Sensitivity of gs to VPD along the gradient of increasing VPD was assessed by Root Mean Square Error (RMSE, σ) of gs data selected the same range of VPD for different trees, respectively. RMSE is a frequently used measure of the differences among several values observed, which represents the sample standard deviation of the differences. The larger σ as a sensitivity parameter related to the higher sensitivity of gs to VPD. demonstrates that the value of σ correlated positively with the concentration of ABA in the leaves of the three trees.Citation37 Therefore, the higher sensitivity of response of gs to VPD was related to the higher concentration of ABA in the leaves of the three trees.
5. Conclusion
We can safely infer from this study that the response of gs to a gradient of increasing VPD can be fitted very well with a Log Normal Model (R2 = 0.845–0.996). Therefore, it can be considered that there is a gs-max in response of gs to VPD in all measured plants which illustrated that there is an optimal range of vapor pressure deficit in the trees. The gs-max-VPD of leaves from each tree species showed little variation among the different seasons during the experimental period, but a positive relationship between gs-max-RH and the mean monthly temperature in 2010 (R2 > 0.81). Two typical stomatal models (the Leuning model and the optimal stomatal model) were used to estimate gs values, and we found a large difference between the predicted data and experimental data in the three tree species in different seasons. The concentration of ABA was positively correlated to sensitivity in response of stomatal conductance to VPD in the leaves of the three tree species in different seasons in Jinan.
However, this study mainly focused on deciduous broad-leaved tree species and did not have enough energy and time to discuss other kinds of plants, such as herbs, conifers and so on. Therefore, the response characteristics of other kinds of plants need to be further studied and validated. At the same time, the meaning of parameters in nonlinear simulation formula of gs to VPD needs further study and discussion.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Dr. Pamela Holt very much for improving the English-writing of the manuscript.
Additional information
Funding
References
- Berry JA, Beerling DJ, Franks PJ. Stomata: key players in the Earth system, past and present. Curr Opin Plant Biol. 2010;13:1–10. doi:10.1016/j.pbi.2010.04.013.
- Smith NG, Keenan TF, Colin PI, Wang H, Wright IJ, Niinemets Ü, Crous KY, Domingues TF, Guerrieri R, Ishida FY, et al. Global photosynthetic capacity is optimized to the environment. Ecol Lett. 2019;22:506–517.
- Sperry JS, Venturas MD, Anderegg WRL, Mencuccini M, Mackay DS, Wang Y, Love DM. Predicting stomatal responses to the environment from the optimization of photosynthetic gain and hydraulic cost. Plant Cell Environ. 2017;40(6):816–830. doi:10.1111/pce.12852.
- Zhang H, Pan CZ, Gu SH, Ma QM, Zhang, Yi Q, Li X, Shi K. Stomatal movements are involved in elevated CO2-mitigated high temperature stress in tomato. Physiol Plant. 2019;165(3):569–583. doi:10.1111/ppl.12752.
- Schachtman DP, Goodger JQD. Chemical root to shoot signaling under drought. Trends Plant Sci. 2008;13:281–287. doi:10.1016/j.tplants.2008.04.003.
- Shang Y, Li M, Ding B, Niu H, Yang ZN, Chen XQ, Cao GY, Xie XD. Advances in auxin regulation of plant stomatal development. Chin Bull Bot. 2017;52:235–240.
- Tanaka Y, Nose T, Jikumaru Y, Kamiya Y. ABA inhibits entry into stomatal-lineage development in Arabidopsis leaves. Plant J. 2013;74:448–457. doi:10.1111/tpj.2013.74.issue-3.
- Ball JT, Woodrow IE, Berry JA. A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions. In: Biggins J editor. Progress in photosynthesis research. Springer; 1987. p. 221–224.
- Dewar RC. The Ball–Berry–Leuning and Tardieu–Davies stomatal models: synthesis and extension within a spatially aggregated picture of guard cell function. Plant Cell Environ. 2002;25(11):1383–1398. doi:10.1046/j.1365-3040.2002.00909.x.
- Farquhar GD, von Caemmerer S, Berry JA. Models of photosynthesis. Plant Physiol. 2001;125:42–45. doi:10.1104/pp.125.1.42.
- Tuzet A, Perrier A, Leuning R. A coupled model of stomatal conductance, photosynthesis and transpiration. Plant Cell Environ. 2003;26:1097–1116. doi:10.1046/j.1365-3040.2003.01035.x.
- Woodruff DR, Meinzer FC, McCulloh KA. Height-related trends in stomatal sensitivity to leaf-to-air vapour pressure deficit in a tall conifer. J Exp Bot. 2010;61(1):203–210. doi:10.1093/jxb/erp291.
- Wang SS, Yang Y, Trishchenko AP, Barr AG, Black TA, McCaughey H. Modeling the response of canopy stomatal conductance to humidity. J Hydrometeorol. 2009;10(2):521–532. doi:10.1175/2008JHM1050.1.
- Jarvis PG. The interpretation of the variations in leaf water potential and stomatal conductance found in canopies in the field. Philos Trans R Soc Lond B Biol Sci. 1976;273(927):593–610. doi:10.1098/rstb.1976.0035.
- Leuning R. A critical appraisal of a combined stomatal photosynthesis model for C3 plants. Plant Cell Environ. 1995;18:339–355. doi:10.1111/pce.1995.18.issue-4.
- Cowan IR, Farquhar GD. Stomatal function in relation to leaf metabolism and environment. Symposia Society for Experimental Biology. 1977;31:471–505.
- Wang YH, Zhou GS. Analysis and quantitative simulation of stomatal conductance of Aneurolepidium chinense. Acta Phytoecologica Sin. 2000;24:739–743.
- Liu YH, Gao Q, Jia HK. Leaf-scale drought resistance and tolerance of three plant species in a semi-arid environment: application and comparison of two stomatal conductance models. J Plant Ecol. 2006;30(1):64–70. doi:10.17521/cjpe.2006.0009.
- Shi XH, Chen ZY, Liu KY, Yang GS, Zhong XH. Effects of different relative humidities on physiological activities of wild grape. Chin J Eco-Agri. 2005;13:65–67.
- Soni DK, Ranjan S, Singh R, Khare PB, Pathre UV, Shirke PA. Photosynthetic characteristics and the response of stomata to environmental determinants and ABA in Selaginella bryopteris, a resurrection spike moss species. Plant Sci. 2012;191:43–52. doi:10.1016/j.plantsci.2012.04.011.
- Perez TM, Feeley KJ. Increasing humidity threatens tropical rainforests. Front Ecol Evol. 2018;6:68. doi:10.3389/fevo.2018.00068.
- Slot M, Winter K. In situ temperature response of photosynthesis of 42 tree and liana species in the canopy of two Panamanian lowland tropical forests with contrasting rainfall regimes. New Phytol. 2017;214(3):1103–1117. doi:10.1111/nph.14469.
- Wright IJ, Dong N, Maire V, Prentice IC, Westoby M, Díaz S, Gallagher RV, Jacobs BF, Kooyman R, Law EA, et al. Global climatic drivers of leaf size. Science. 2017;357(6354):917–921. doi:10.1126/science.aal4760.
- Arbona V, Perez-Clemente RM, Sanchez-Perez AM, Manzi M, Zandalinas SI, Vives V, Gomez-Cadenas A. Abscisic acid: a versatile phytohormone in plant signaling and beyond. Curr Protein Pept Sci. 2015;16(5):413–434. doi:10.2174/1389203716666150330130102.
- Merilo E, Jalakas P, Laanemets K, Mohammadi O, Hrak H, Kollist H, Brosche M. Abscisic acid transport and homeostasis in the context of stomatal regulation. Mol Plant. 2015;8(9):1321–1333. doi:10.1016/j.molp.2015.06.006.
- Min MK, Choi EH, Kim JA, Yoon IS, Han S, Lee Y, Lee S, Kim BG. Two clade a phosphatase 2Cs expressed in guard cells physically interact with abscisic acid signaling components to induce stomatal closure in rice. Rice. 2019;12(1):1–13. doi:10.1186/s12284-019-0297-7.
- Qin T, Tian QZ, Wang GF, Xiong LM. Lower temperature 1 enhances ABA responses and plant drought tolerance by modulating the stability and localization of C2-Domain ABA-related proteins in Arabidopsis. Mol Plant. 2019;12(9):1243–1258. doi:10.1016/j.molp.2019.05.002.
- Tao HC, Xu S, Fu W, He XY, Chen W, Ma CL, Li Y, Wu X. Effects of elevated O3 concentration and drought on photosynthetic physiological characteristics of Syringa oblate leaves. Jiangsu Agri Sci. 2019;47:186–190.
- Wang SC, Li C, ShiS. G, Ma FW. Effects of exogenous aba on leaf anatomy and hormone contents of apple rootstocks. Agri Res Arid Areas. 2019;37:31–40.
- Bunce JA. Does transpiration control stomatal responses to water vapour pressure deficit?. Plant Cell Environ. 1996;19:131–135.
- Snaith PJ, Mansfield TA. Stomatal sensitivity to abscisic acid: can it be defined? Plant Cell Envi. 1982;5(4):309–311. doi:10.1111/pce.1982.5.issue-4.
- Tanaka Y, Sano T, Tamaoki M, Nakajima N, Kondo N, Hasezawa S. Cytokinin and auxin inhibit abscisic acid-induced stomatal closure by enhancing ethylene production in Arabidopsis. J Exp Bot. 2006;57(10):2259–2266. doi:10.1093/jxb/erj193.
- Acharya BR, Assmann SM. Hormone interactions in stomatal function. Plant Mol Biol. 2009;69(4):451–462. doi:10.1007/s11103-008-9427-0.
- Bi GH, Wu GX, Zhang GZ. Annual variation of IAA and ABA contents in young apple tree. J Shandong Agri Univ. 1995;2:98–102.
- Börner A, Plaschke J, Korzun V, Worland AJ. The relationships between the dwarfing genes of wheat and rye. Euphytica. 1996;89(1):69–75. doi:10.1007/BF00015721.
- Hoffmann BS, Kende H. On the role of abscisic-acid and gibberellin in the regulation of growth in rice. Plant Physiol. 1999;99:1156–1161. doi:10.1104/pp.99.3.1156.
- Li J, Li XM. Response of stomatal conductance of two tree species to vapor pressure deficit in three climate zones. J Arid Land. 2014;6(6):771–781. doi:10.1007/s40333-014-0030-8.
- Dias MC, Brüggemann W. Differential inhibition of photosynthesis under drought stress inflaveriaspecies with different degrees of development of the C4 syndrome. Photosynthetica (Prague). 2007;45(1):75–84. doi:10.1007/s11099-007-0012-6.
- Liu HM, Long CR, Li JX, Fu XM, Zhou DG, Gao JY, Dong MC, Yue JQ. A study on photosynthetic characteristics and fruiting performance of three lemon varieties in dry-hot valley regions in Yunnan province. J Fruit Sci. 2017;34:59–68.
- Wu XL, Tang Z, Wu Y, Cao JW, Li Q, Ma B, Chen YZ, Sun MH, Li JJ. Diurnal variation regulations of photosynthetic characteristics in two Camellia oleifera cultivars and six related C. oleifera species. Non-wood for Res. 2019;37:101–109.
- Huang ZY, Dong XJ. Primary studies on the daily dynamic changes of photosynthesis and transpiration of Salix psammophila. Acta Bot Boreali-Occidentalia Sin. 2002;22:817–823.
- Weng XY, Jiang DA. Regulation of rubisco activity and diurnal changes of photosynthetic rate in rice by ecology factors. J Zhejiang Univ. 2002;28:387–391.
- Yang MH, Li ZH, Huang LQ, Yang Y. Photosynthesis change in a day of Ginkgo Biloba. Nonwood Forest Res. 2004;22:15–18.
- Zhao P, Zeng XP, Peng SL, Sun GT. Daily variation of gas exchange, stomatal conductance and water use efficiency in summer leaves of Ormosia Pinnata. J Trop Subtropical Bot. 2000;8:35–42.
- Héroult A, Lin YS, Bourne A, Medlyn BE, Ellsworth DS. Optimal stomatal conductance in relation to photosynthesis in climatically contrasting Eucalyptus species under drought. Plant Cell Environ. 2013;36:262–274. doi:10.1111/j.1365-3040.2012.02570.x.
- Mdelyn BE, Duursma RA, Eamus D, Ellsworth DS, Prentice IC, Barton CVM, Crous KY, Angelis DE, Freeman DE, Wingate PML. Reconciling the optimal and empirical approaches to modelling stomatal conductance. Glob Chang Biol. 2011;17:2134–2144. doi:10.1111/j.1365-2486.2010.02375.x.
- Fu L, Wang ML, Gao JG, Sheng F, Su XR. Annual dynamic patten of four endogenous hormones in young apple trees. J Shandong Agri Univ. 2000;31:180–182.
- Bland JM, Altman DG. Statistics notes: measurement error. Br Med J. 1996;312(7047):1654. doi:10.1136/bmj.312.7047.1654.
- Morison JI, Gifford RM. Stomatal sensitivity to carbon dioxide and humidity a comparison of two C3 and two C4 grass species. Plant Physiol. 1983;71:789–796.
- Rosenberg NJ, Blad BL, Verma SB. Microclimate: the biological environment, 2nd ed. Hoboken: Wiley; 1983.
- Chang JC, Lin TZ. Gas exchange in litchi under controlled and field conditions. Sci Hortic (Amsterdam). 2007;114:268–274. doi:10.1016/j.scienta.2007.06.023.
- Eamus D, Taylor DT, Maccinis-Ng CMO, Shanahan S, DeSilva L. Comparing model predictions and experimental data for the response of stomatal conductance and guard cell turgor to manipulations of cuticular conductance, leaf-to-air vapour pressure difference and temperature: feedback mechanisms are able to account for all observations. Plant Cell Environ. 2008;31:269–277.
- Yang SQ, Yang ZQ, Wang L, Li J, Zhang MY, Li KW. Effect of high humidity and high temperature interaction on photosynthetic characteristics of greenhouse tomato crops. Chin J Ecol. 2018;37:57–63.
- Zhu YQ, Yang ZQ. Effects of high temperature and high humidity on stomatal and photosynthesis characteristics of grape leaves in greenhouse. North Hortic. 2017;23:94–101.
- Aronne G, De Micco V. Seasonal dimorphism in the Mediterranean Cistus incanus L subsp.incanus. Ann Bot. 2001;87:789–794. doi:10.1006/anbo.2001.1407.
- Arve LE, Terfa MT, Gislerød HR, Olsen JE, Torre S. High relative air humidity and continuous light reduce stomata functionality by affecting the ABA regulation in rose leaves. Plant Cell Environ. 2013;36:382–392. doi:10.1111/j.1365-3040.2012.02580.x.
- Cai B. Comparative study on biological characteristics of Plukenetia volubilis L. under different sites condition. Haikou: HaiNan University; 2018.
- Fanourakis D. Stomatal response characteristics as affected by long-term elevated humidity levels. PLoS One. 2011;1-182.
- Han L, He J, Qi TY, Tian J, Zhan XL. Responses and modeling of canopy stomatal conductance of platycladus orientalis to environmental factors in hedong sandy land, ningxia. Chin J Ecol. 2018;37:2862–2868.
- Hetherington AM, Woodward FI. The role of stomata in sensing and driving environmental change. Nature. 2003;424:901–908. doi:10.1038/nature01843.
- Zhang ZZ, Zhao P, Zhao XH, Zhang JX, Zhu LW, Ouyang L, Zhang XY. Impact of environmental factors on the decoupling coefficient and the estimation of canopy stomatal conductance for ever-green broad-leaved tree species. Chin J Plant Ecol. 2018;42(12):1179–1191. doi:10.17521/cjpe.2018.0176.
- Prenger JJ, Ling PP. Greenhouse condensation control understanding and using vapor pressure deficit (VPD), Fact Sheet Extension. Columbus: The Ohio State University. AEX-804; 2001. p. 1–4
- Huang J, Chen ZF, Yin LY, Li MQ, Wang LH, Teng WC. Effects of three plant exogenous hormones on the biomass,chlorophyll fluorescence parameters,and photosynthetic characteristics of tabebuia chrysantha seedlings. Plant Sci J. 2018;36:745–754.
- Jia FL, Wang CP, Liu S, Jiao ZY, Yin WL, Xia XL. Effects of exogenous br and IAA on drought tolerance of populus deltoides ×p. nigra. J Beijing For Univ. 2017;39:31–39.
- Le J, Liu XG, Yang KZ, Chen XL, Zou JJ, Wang HZ, Wang M, Vanneste S, Miyo M, Tasaka M, et al. Auxin transport and activity regulate stomatal patterning and development. Nat Commun. 2014;5:3090. doi:10.1038/ncomms4090.
- Wei DZ, Dai XB, Xu XM, Zhang RX. Several hypotheses on the mechanism of the plant leaf senescence. Guihaia. 1998;18:89–96.
- Yan WY, Ye SH, Dong YJ, Jin QS, Zhang XM. Research progress related to plant leaf senescence. Crops. 2010;36:4–9.
- Ma XL, LI HS. Review of the mechanisms of hybrid rice early aging. Hunan Agr Sci. 2007;3:59–61.
- Liang JS. Effects of ABA and vapour pressure deficit on stomatal conductance of sunflower leaves. J YangZhou Univ. 1999;2:41–45.