 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Metabolic efficiency of root growth is a crucial physiological parameter, contributing to the amount of photosynthate that plants need to invest into soil exploration. Common measurements of metabolic efficiency usually rely on CO2 respiration measurements with the underlying assumption that all metabolic processes are taking place under aerobic conditions. In this conceptual paper, we introduce energy use efficiency based on the quantification of heat dissipation and energy fluxes as an alternative metric to quantify the metabolic efficiency of root growth. In a theoretical framework, we adopted recently published heat dissipation data from wheat seedlings and show that energy use efficiency decreases in response to (i) soil hypoxia and (ii) increased soil penetration resistance. In contrast to traditional CO2 respiration measurements, heat dissipation measurements account for both aerobic as well as anaerobic respiration in growing roots. Hence, we advocate that the quantification of heat dissipation provides a more complete picture of the metabolic efficiency of root growth than CO2 respiration measurements alone. We therefore propose that energy use efficiency should be included in future studies assessing the metabolic efficiency of root growth.
Main text
Root growth is vital for plants to acquire water and nutrients but it requires substantial amounts of energy and thus photosynthate. Such an investment into belowground growth can significantly limit crop productivity, in particular under low soil fertility. Improving metabolic efficiency of root growth is therefore seen as a promising strategy to adapt crops to less fertile soils such as dry, flooded and compacted soils, and soils with low nutrient availability.Citation1,Citation2 Metabolic efficiency is defined as the proportion between resources allocated to anabolic processes such as growth and catabolic processes such as respiration.Citation3 Usually metabolic efficiency is assessed by CO2 respiration measurements, which relies on the assumption that all metabolic processes are occurring under aerobic conditions.Citation4 However, as soils are regularly hypoxic,Citation5–Citation7 it is likely that the metabolism of growing roots is at least partially anaerobic. Recently, soil scientists have shown increased interest in quantifying the metabolic efficiency of soil microorganisms using a bioenergetics approach based on heat dissipation and energy fluxes.Citation4,Citation8 Compared to CO2 respiration measurements, heat dissipation recordings account for all metabolic processes. Metabolic efficiency is then expressed as energy use efficiency, referring to the proportion between energy allocated to anabolic processes such as growth and catabolic processes such as aerobic and anaerobic respiration.
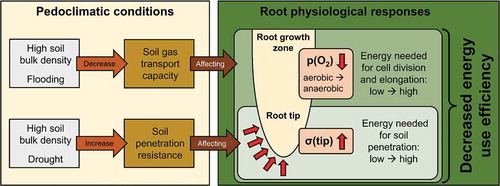
In the conceptual framework presented here, we outline the use of energy use efficiency to assess the metabolic efficiency of root growth using soil hypoxia and high soil penetration resistance as case examples. Hypoxic conditions typically occur in flooded and dense soil, resulting in lower cellular oxygen concentration and a shift from aerobic to anaerobic respiration in the root growth zone.Citation9–Citation11 Due to this shift, cell division and cell elongation require greater amounts of energy (). High penetration resistance characterizes dry and dense soil and imposes greater mechanical stress on the growing root tip, which increases the energy needed to penetrate soilCitation9,Citation12,Citation13 (). Ultimately, low cellular oxygen concentration and high mechanical stress at the root tip increase the energy costs of root growth,Citation2,Citation9 which decreases the energy use efficiency of root growth (). We used recently published data on root growth and heat dissipation of wheat (Triticum aestivum L.) seedlings (n = 240) that were pre-germinated for 72 h followed by a 24 h growth period under three different levels of soil penetration resistance2. Energy allocated to all catabolic processes in the growing root (Qc) was calculated as:
Figure 1. Conceptual overview illustrating relationships between pedoclimatic conditions and energy use efficiency of root growth. High bulk density and flooding decrease soil gas transport capacity, resulting in (i) decreased cellular oxygen concentration (p(O2)) in the root growth zone and (ii) a shift from aerobic to anaerobic metabolism. Furthermore, high bulk density and drought increase soil penetration resistance, leading to higher mechanical stress at the growing root tip (σ(tip)). Energy requirements of root growth increase in response to low p(O2) and high σ(tip), resulting in higher amounts of energy allocated to catabolic than anabolic processes and thus in a decrease of energy use efficiency.
where Qm [kJ] denotes the cumulative heat measured during 24 h by isothermal calorimetry2. CRav represents the average calorespirometric ratio of carbohydrates such as glucose under aerobic conditions of 30 kJ g−1 CO2-C. CRc denotes the calorespirometric ratio of all catabolic processes in the growing root, which was set to either 30 kJ g−1 CO2-C or 60 kJ g−1 CO2-C to represent fully aerobic and partially anaerobic conditions, respectively.Citation14,Citation15 A higher calorespirometric ratio resulted in increased Qc, while soil penetration resistance did not affect Qc (). Energy allocated to anabolic processes (Qa [kJ]), i.e. root energy content, was calculated using the molar combustion enthalpyCitation16 (∆HC gluc. = 2813.6 kJ mol−1), the molar mass (Mgluc. = 180.16 g mol−1) and the carbon content (Cgluc. = 0.4 g g−1) of glucose as:
Figure 2. Effects of calorespirometric ratio and soil penetration resistance (PR) on (a) the energy allocated to root respiration (catabolic energy Qc; Eq. 1), (b) the energy allocated to root growth (anabolic energy Qa; Eq. 2), and (c) the ratio between Qc and Qa. Calorespirometric ratio of 30 and 60 kJ g−1 CO2-C represent fully aerobic and partially anaerobic conditions, respectively. Calculations are based on mean values (n = 5) of 16 wheat genotypes taken from Colombi et al.Citation2.
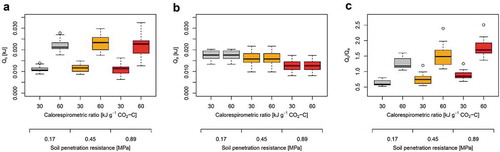
where Vbm [cm3] denotes root volume2 and ρbm represents the tissue density of 1 g cm−3. The ratio between root dry weight (dw) and root fresh weight (fw)Citation17 was set to 0.1 g g−1 and root carbon content (Cbm)Citation18 was set to 0.38 g g−1. Since Qa is independent from the calorespirometric ratio and we assumed constant root biomass under aerobic and partially anaerobic conditions (Eq. 2), Qa did not change upon increased calorespirometric ratio. However, Qa decreased with increasing soil penetration resistance due to decreased root growth2 (). Calculating the proportion between Qc and Qa shows how soil hypoxia and increased soil penetration resistance affect energy allocation. More energy was allocated to root respiration than to growth in response to soil hypoxia, i.e. upon an increase in the calorespirometric ratio, and upon greater penetration resistance (). Following previous definitions of metabolic efficiency3, energy use efficiency (EUE) was then calculated as:
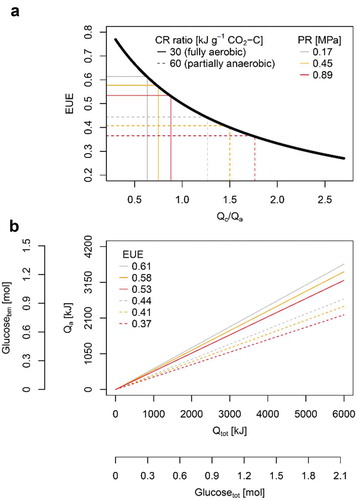
Due to the increase of the proportion between Qc and Qa, energy use efficiency decreased by approximately 30% when the calorespirometric ratio increased from 30 kJ g−1 CO2-C to 60 kJ g−1 CO2-C. Accordingly, energy use efficiency decreased between 6% and 18% upon higher soil penetration resistance (). Moreover, energy use efficiency differed substantially among wheat genotypes (), indicating that there is genotypic diversity in energy use efficiency within a single species. As depicted in Eq. 3, the proportion of energy allocated to root growth (Qa) relative to the total energy required for growth (Qtot) decreases proportionally with energy use efficiency. Finally, carbohydrate-equivalents were derived from energy values using the molar combustion enthalpy of 2813.6 kJ mol−1 glucose. Using carbohydrate-equivalents, it becomes evident that plants need to invest more carbohydrates into root growth when energy use efficiency decreases ().
Figure 3. (a) Energy use efficiency (EUE; Eq. 3) as a function of the ratio between energy allocated to root respiration (catabolic energy Qc; Eq. 1) and root growth (anabolic energy Qa; Eq. 2) at different calorespirometric ratio (CR ratio) and levels of soil penetration resistance (PR). CR ratio of 30 and 60 kJ g−1 CO2-C represent fully aerobic and partially anaerobic conditions, respectively. (b) Qa as a function of the total energy required for root growth (Qtot; Eq. 3) at different EUE. Carbohydrate equivalents for Qa (Glucosebm) and Qtot (Glucosetot) were calculated using the molar combustion enthalpy of 2813.6 kJ mol−1 glucose. CR ratio and soil penetration resistance are indicated by line style and color, respectively. All calculations are based on mean values of 16 wheat genotypes and five replicates (n = 80) taken from Colombi et al.Citation2.
With this conceptual paper, we show that the metabolic efficiency of plant root growth can be assessed through the quantification of energy fluxes. Energy use efficiency is a suitable metric to determine the effects of soil hypoxia and increased soil penetration resistance on the metabolic costs of soil exploration (). The calorespirometric ratio associated with the metabolism in growing roots is a crucial parameter when determining energy use efficiency, particularly under hypoxic conditions. In the present framework, we assumed two theoretical calorespirometric ratios (Eq. 1; CRc) to illustrate the effects of soil hypoxia on the metabolic efficiency of root growth. Similarly, we calculated the anabolic energy allocated to root growth (Qa), i.e. root energy content, based on root volume data, estimated root dry matter and carbon content, and the molar combustion enthalpy of glucose (Eq. 2). Our conceptual framework can therefore be further developed. The calorespirometric ratio of all catabolic processes in growing roots can be quantified directly by combining heat dissipation recordings with CO2 respiration measurements.Citation4,Citation14 Moreover, the energy content of roots can also be assessed using bomb calorimetryCitation19 together with measurements of root dry weight and root carbon content. In doing so, plant responses to soil-borne stress such as compaction, flooding or drought and their influences on energy use efficiency of root growth can be quantified directly.
Because soil hypoxia occurs regularly due to impeded gas exchange through the soil profile,Citation5–Citation7 respiratory processes in roots are likely to be at least partially anaerobic. Hence, the most significant advantage of the proposed framework is that heat dissipation compared to traditionally used CO2 respiration measurements accounts for both aerobic and anaerobic respiration processes. We therefore suggest that energy use efficiency should be considered when aiming to improve the metabolic efficiency of root growth.
Acknowledgments
The authors express their gratitude to the Lantmännen Research Foundation (grant number: 2017H022) and the Royal Swedish Academy of Agriculture and Forestry (KSLA; grant number: GFS2017-0118) for financial support. Furthermore, T. Colombi received funding from the Swedish Governmental Agency for Innovation Systems (Vinnova; grant number: 2018-02346) and A.M. Herrmann received funding from the Swedish Research Council for Sustainable Development (Formas; grant number: 2017-00932), which is greatly acknowledged.
Additional information
Funding
References
- Lynch JP. Root phenes that reduce the metabolic costs of soil exploration: opportunities for 21st century agriculture. Plant Cell Environ. 2015;38:1–4.
- Colombi T, Herrmann AM, Vallenback P, Keller T. Cortical cell diameter is key to energy costs of root growth in wheat. Plant Physiol. 2019;180:2049–2060.
- Geyer KM, Dijkstra P, Sinsabaugh R, Frey SD. Clarifying the interpretation of carbon use efficiency in soil through methods comparison. Soil Biol Biochem. 2019;128:79–88.
- Herrmann AM, Bölscher T. Simultaneous screening of microbial energetics and CO2 respiration in soil samples from different ecosystems. Soil Biol Biochem. 2015;83:88–92.
- Weisskopf P, Reiser R, Rek J, Oberholzer HR. Effect of different compaction impacts and varying subsequent management practices on soil structure, air regime and microbiological parameters. Soil Tillage Res. 2010;111:65–74.
- Cannell RQ, Belford RK, Gales K, Thomson RJ, Webster CP. Effects of waterlogging and drought on winter wheat and winter barley grown on a clay and a sandy loam soil - I. Crop growth and yield. Plant Soil. 1984;80:53–66.
- Buyanovsky GA, Wagner GH. Annual cycles of carbon dioxide level in soil air. Soil Sci Soc Am J. 1983;47:1139–1145.
- Arcand MM, Levy-Booth DJ, Helgason BL. Resource legacies of organic and conventional management differentiate soil microbial carbon use. Front Microbiol. 2017;8:1–17.
- Colombi T, Keller T. Developing strategies to recover crop productivity after soil compaction—A plant eco-physiological perspective. Soil Tillage Res. 2019;191:156–161.
- Bailey-Serres J, Lee SC, Brinton E. Waterproofing crops: effective flooding survival strategies. Plant Physiol. 2012;160:1698–1709.
- Fukao T, Bailey-Serres J. Plant responses to hypoxia - Is survival a balancing act? Trends Plant Sci. 2004;9:449–456.
- Bengough AG, McKenzie BM, Hallett PD, Valentine TA. Root elongation, water stress, and mechanical impedance: a review of limiting stresses and beneficial root tip traits. J Exp Bot. 2011;62:59–68.
- Ruiz S, Straub I, Schymanski SJ, Or D. experimental evaluation of earthworm and plant root soil penetration–cavity expansion models using cone penetrometer analogs. Vadose Zo J. 2016;15.
- Gnaiger E, Kemp RB. Anaerobic metabolism in aerobic mammalian cells: information from the ratio of calorimetric heat flux and respirometric oxygen flux. Biochim Biophys Acta. 1990;1016:328–332.
- Hansen LD, Macfarlane C, McKinnon N, Smith BN, Criddle RS. Use of calorespirometric ratios, heat per CO2 and heat per O2, to quantify metabolic paths and energetics of growing cells. Thermochim Acta. 2004;422:55–61.
- Herrmann AM, Coucheney E, Nunan N. Isothermal microcalorimetry provides new insight into terrestrial carbon cycling. Environ Sci Technol. 2014;48:4344–4352.
- Shipley B, Vu -T-T. Dry matter content as a measure of dry matter concentration in plants and their parts. New Phytol. 2001;153:359–364.
- Ma S, He F, Tian D, Zou D, Yan Z, Yang Y, Zhou T, Huang K, Shen H, Fang J. Variations and determinants of carbon content in plants: a global synthesis. Biogeosciences. 2018;15:693–702.
- Bölscher T, Paterson E, Freitag T, Thornton B, Herrmann AM. Temperature sensitivity of substrate-use efficiency can result from altered microbial physiology without change to community composition. Soil Biol Biochem. 2017;109:59–69.
