ABSTRACT
Plant small RNA (sRNA)-mediated gene expression has a conserved role in regulating plant growth, development, and immunity. Heterologous expression of sRNA contributes to determining whether the function of sRNA is conservative or independent. We recently characterized the Tourist-miniature inverted-repeat transposable element (MITE)-derived siR109944 had a conserved function that enhanced susceptibility to Rhizoctonia solani infection by affecting auxin homeostasis in rice and Arabidopsis. To ascertain whether the function of rice siR109944 has a broad-spectrum immunity in Arabidopsis, we infected Arabidopsis with a variety of fungal pathogens. Overexpression of siR109944 in Arabidopsis increased susceptibility to Botrytis cinerea, Sclerotinia sclerotium, and Verticillium dahliae infection. Further studies found that Arabidopsis auxin-related miRNAs were suppressed in siR109944 OE. Our results demonstrated that overexpression of rice siR109944 in Arabidopsis affected immune responses to multiple pathogens by inhibiting auxin-related miRNA expression in planta.
sRNA-mediated RNA interference (RNAi) is a conserved immune regulatory mechanism in most eukaryotes, which is involved in host immunity and pathogen virulence.Citation1–Citation3 Many studies have identified the role of sRNAs in rice and Arabidopsis in plant immunity; however, the heterologous regulating function of sRNAs has scarcely been studied. Heterologous gene expression utilizes gene resources and can be useful for the screening of disease-resistant breeding and increasing the cognition of immune regulatory responses among different species. For example, heterologous expression of Vitis amurensis VaERF20 in Arabidopsis improves resistance to Botrytis cinerea and Pseudomonas syringae pv. tomato DC3000.Citation4 Host-induced gene silencing is a combination of heterologous expression and RNAi technology to enhance host resistance by constructing transgenic plants by targeting important pathogenic genes that are necessary for growth, development, and pathogenicity.Citation5–Citation7
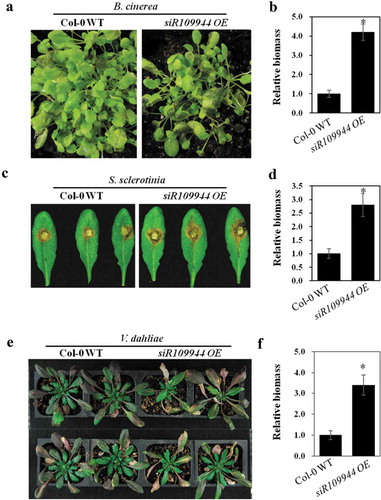
Based on our present study, we found that rice Tourist-miniature inverted-repeat transposable element (MITE)-derived siR109944 suppressed plant immunity to sheath blight, and overexpression of siR109944 in Arabidopsis could enhance susceptibility to Rhizoctonia solani by modulating auxin homeostasis.Citation8 To explore whether overexpression of siR109944 enhanced the broad-spectrum immune response in Arabidopsis, the OE line with a higher expression level was selected for this study.Citation8 We first inoculated siR109944 OE and Col-0 WT seedlings with B. cinerea, a destructive fungus. Arabidopsis plants with siR109944 overexpression were more susceptible to B. cinerea infection than Col-0 WT plants (,)). In addition to B. cinerea, Sclerotinia sclerotiorum is another serious plant pathogenic fungus that can cause white mold disease.Citation9 Verticillium dahliae causes verticillium wilt in many plant species and causes Arabidopsis leaves to curl and discolor.Citation10 Then, siR109944 OE and Col-0 WT plant roots and leaves were inoculated with V. dahliae spores and S. sclerotiorum mycelium plugs, respectively. Compared to Col-0 WT plants, we found that siR109944 OE plants displayed more sensitive disease phenotypes caused by S. sclerotium and V. dahliae (–)). It has been shown that auinx is involved in the modulation of various plant defense signals against pathogens, including B. cinerea, S. sclerotium, and V. dahlia.Citation11–Citation13 Collectively, our results demonstrated that the expression of rice siR109944 in Arabidopsis regulated plant broad-spectrum immunity against fungi.
Figure 1. Heterologous expression siR109944 suppresses Arabidopsis immunity against fungal infection (a) The phenotypes of Col-0 WT and siR109944 OE Arabidopsis seedlings were inoculated by foliar spraying with Botrytis cinerea spore suspension, and photos were taken 2 days post-inoculation (dpi). (b) The B. cinerea relative DNA content (relative biomass) was measured using quantitative PCR, and error bars represent the standard deviation (s.d.) of three technical replicates. Asterisks indicate significant differences. (c) The phenotypes of Sclerotinia sclerotiorum infect Col-0 WT and siR109944 OE transgenic Arabidopsis, and photos were taken at 2 dpi. (d) The relative biomass of S. sclerotiorum was measured using quantitative PCR, and error bars represent the s.d. of three technical replicates. Asterisks indicate significant differences. (e) Arabidopsis Col-0 WT and siR109944 OE were infected by Verticillium dahliae spores using the root dipping method, and photos were taken at 10 dpi. (f) Relative biomass of V. dahliae was detected by quantitative PCR, and error bars represent the s.d. of three technical replicates. The experiments were repeated three times with similar results. Asterisks indicate significant differences (*P < .05).
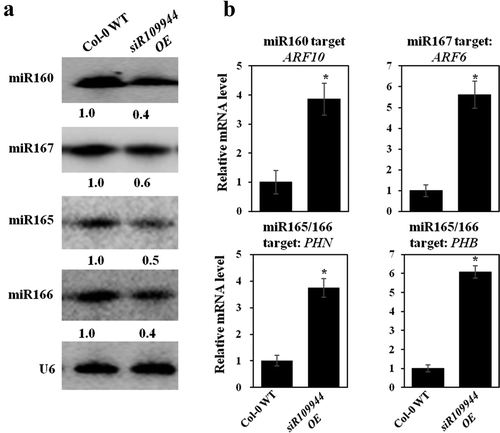
Auxin plays an important role in regulating many aspects that affect Arabidopsis growth and development,Citation14,Citation15 and some miRNAs are involved in the regulation of auxin. Since the expression of siR109944 could affect Arabidopsis disease immunity by affecting the auxin-regulated gene expression,Citation8 we explored whether overexpression of siR109944 could alter auxin-related miRNA expression in Arabidopsis. Overexpression of miR165 alters the expression of genes involved in auxin signaling and vascular development of Arabidopsis.Citation16 Repression of AUXIN RESPONSE FACTOR10 (ARF10) and ARF17 by miR160 is critical for seed germination and post-germination stages.Citation17,Citation18 Auxin-related miRNAs, including miR160, miR167, and miR165/166 in Col-0 WT and siR109944 OE, were detected in Arabidopsis by northern blot. The results showed that the expression levels of these miRNAs were more down-regulated in siR109944 OE than in Col-0 WT plants ()). Moreover, the target genes of these miRNAs had the opposite expression levels as the related miRNAs, including ARF10 (miR160), ARF6 (miR167), PHB, and PHN (miR165/166) in Col-0 WT and siR109944 OE plants ()). It was speculated that overexpression of siR109944 enhanced disease susceptibility against multiple fungi might be due to the inhibition of auxin-related miRNA expression in Arabidopsis.
Figure 2. Heterologous expression siR109944 affects Arabidopsis auxin-related miRNA expression. (a) The expression levels of auxin-related miRNAs (miR160, miR167, and miR165/166) in Col-0 WT and siR109944 OE plants by northern blot. Values below each section represent the relative abundance (RA) of miRNA normalized to U6. (b) Quantitative PCR analysis of the relative expression of the miR160 target gene ARF10, miR167 target gene ARF6, and miR165/166 target genes PHN and PHB in Col-0 WT and siR109944 OE plants. The internal reference was AtActin. The experiments were repeated three times with similar results. Asterisks indicate significant differences (*P < .05).
RNA interference (RNAi) is a regulatory mechanism for gene silencing by which siRNAs guide RNA-induced silencing complexes (RISC) to cleave homologous transcripts.Citation19 Therefore, the RNAi mechanism is conserved among different species. We found that the target genes of siR109944 in rice had highly homologous genes in Arabidopsis, but these genes were not the target of siR109944 in Arabidopsis.Citation8 Though the regulatory networks of small RNAs vary among plant species and are highly diverse, siRNA pathways have adopted new functions to create novel plant morphologies and immune response by heterologous expression.
Disclosure of Potential Conflicts of Interest
There are no potential conflicts of interest to disclose.
Additional information
Funding
References
- Baulcombe D. RNA silencing in plants. Nature. 2004;431(7006):1–4. PMID: 15372043. doi:10.1038/nature02874.
- Vaucheret H. Post-transcriptional small RNA pathways in plants: mechanisms and regulations. Genes Dev. 2006;20:759–771. PMID: 16600909 http://www.genesdev.org/cgi/doi/10.1101/gad.1410506
- Baldrich P, San Segundo B. MicroRNAs in rice innate immunity. Rice. 2016;9(1):6. PMID: 26897721. doi:10.1186/s12284-016-0078-5.
- Wang M, Zhu Y, Han R, Yin W, Guo C, Li Z, Wang X. Expression of Vitis amurensis VaERF20 in Arabidopsis thaliana improves resistance to Botrytis cinerea and Pseudomonas syringae pv. Tomato DC3000. Int J Mol Sci. 2018;19(3):696. PMID: 29494485. doi:10.3390/ijms19030696.
- Qi T, Guo J, Peng H, Liu P, Kang Z, Guo J. Host-induced gene silencing: a powerful strategy to control diseases of wheat and barley. Int J Mol Sci. 2019;20(1):206. PMID: 30626050. doi:10.3390/ijms20010206.
- Shivakumara TN, Chaudhary S, Kamaraju D, Dutta TK, Papolu PK, Banakar P, Sreevathsa R, Singh B, Manjaiah KM, Rao U, et al. Host-induced silencing of two pharyngeal gland genes conferred transcriptional alteration of cell wall-modifying enzymes of meloidogyne incognita vis-a-vis perturbed nematode infectivity in eggplant. Front Plant Sci. 2017;8:473. PMID: 28424727. doi:10.3389/fpls.2017.00473.
- Govindarajulu M, Epstein L, Wroblewski T, Michelmore RW. Host-induced gene silencing inhibits the biotrophic pathogen causing downy mildew of lettuce. Plant Biotechnol J. 2015;13(7):875–883. PMID: 25487781. doi:10.1111/pbi.12307.
- Qiao L, Zheng L, Sheng C, Zhao H, Jin H, Niu D. Rice siR109944 suppresses plant immunity to sheath blight and impacts multiple agronomic traits by affecting auxin homeostasis. Plant J. 2020. PMID: 31923320. doi:10.1111/tpj.14677.
- Bolton MD, Thomma BP, Nelson BD. Sclerotinia sclerotiorum (Lib.) de Bary: biology and molecular traits of a cosmopolitan pathogen. Mol Plant Pathol. 2006;7(1):1–16. PMID: 20507424. doi:10.1111/j.1364-3703.2005.00316.x.
- Veronese P, Narasimhan ML, Stevenson RA, Zhu JK, Weller SC, Subbarao KV, Bressan RA.. Identification of a locus controlling Verticillium disease symptom response in Arabidopsis thaliana. Plant J. 2003;35(5):574–587. PMID: 12940951. doi:10.1046/j.1365-313X.2003.01830.x.
- Stotz HU, Jikumaru Y, Shimada Y, Sasaki E, Stingl N, Mueller MJ, Kamiya Y. Jasmonate-dependent and COI1-independent defense responses against Sclerotinia sclerotiorum in Arabidopsis thaliana: auxin is part of COI1-independent defense signaling. Plant Cell Physiol. 2011;52(11):1941–1956. PMID: 21937677. doi:10.1093/pcp/pcr127.
- Windram O, Madhou P, McHattie S, Hill C, Hickman R, Cooke E, Jenkins DJ, Penfold CA, Baxter L, Breeze E, et al. Arabidopsis defense against Botrytis cinerea: chronology and regulation deciphered by high-resolution temporal transcriptomic analysis. Plant Cell. 2012;24(9):3530–3557. PMID: 23023172. doi:10.1105/tpc.112.102046.
- Fousia S, Tsafouros A, Roussos PA, Tjamos SE. Increased resistance to Verticillium dahliae in Arabidopsis plants defective in auxin signalling. Plant Pathol. 2018;67(8):1749–1757. PMID. doi:10.1111/ppa.12881.
- Teale WD, Paponov IA, Palme K. Auxin in action: signalling, transport and the control of plant growth and development. Nature Rev Mol Cell Biol. 2006;7(11):847–859. PMID: 16990790. doi:10.1038/nrm2020.
- Zhao Y. Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol. 2010;61(1):49–64. PMID: 20192736. doi:10.1146/annurev-arplant-042809-112308.
- Zhou GK, Kubo M, Zhong R, Demura T, Ye ZH. Overexpression of miR165 affects apical meristem formation, organ polarity establishment and vascular development in Arabidopsis. Plant Cell Physiol. 2007;48(3):391–404. PMID: 17237362. doi:10.1093/pcp/pcm008.
- Liu PP, Montgomery TA, Fahlgren N, Kasschau KD, Nonogaki H, Carrington JC. Repression of AUXIN RESPONSE FACTOR10 by microRNA160 is critical for seed germination and post-germination stages. Plant J. 2007;52(1):133–146. PMID: 17672844. doi:10.1111/j.1365-313X.2007.03218.x.
- Mallory AC, Bartel DP, Bartel B. MicroRNA-directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes. Plant Cell. 2005;17(5):1360–1375. PMID:15829600. doi:10.1105/tpc.105.031716.
- Dunoyer P, Himber C, Voinnet O. DICER-LIKE 4 is required for RNA interference and produces the 21-nucleotide small interfering RNA component of the plant cell-to-cell silencing signal. Nat Genet. 2005;37(12):1356–1360. PMID: 16273107. doi:10.1038/ng1675.
