ABSTRACT
The root endophytic fungus Piriformospora indica plays an important role in increasing abiotic stress tolerance of its host plants. To explore the impact of P. indica on freezing tolerance, Arabidopsis seedlings were co-cultivated with P. indica exposed to −6°C for 6 h. Freezing stress decreased the survival rate, electrolyte leakage, leaf temperature, water potential and chlorophyll fluorescence of Arabidopsis plants in comparison to the controls. P. indica colonizion reduced the negative effects of freezing, and the plants contained also higher amounts of soluble proteins, proline and ascorbic acid during the post-thaw recovery period (4°C; 12 h). In contrast, the H2O2 and malondialdehyde levels were reduced in seedlings colonized by the fungus. The brassinolide (BR) and abscisic acid (ABA) levels dramatically increased and the transcript levels of several crucial freezing-stress related genes (CBFs, CORs, BZR1, SAG1 and PYL6) were higher in inoculated plants during the post-thaw recovery period. Finally, inocculated mutants impaired in the freezing tolerance response (such as ice1 for INDUCER OF CBF EXPRESSION1, a crucial basic helix-loop-helix transcription factor for the cold-response pathway in Arabidopsis, cbf1, −2, −3 for C-REPEAT-Binding Factor, cor47 and −15 for COLD-REGULATED and siz1 encoding the SUMO E3 LIGASE) showed better survival rates and higher expression levels of freezing-related target genes after freezing compared to the inocculated controls. Our results demonstrate that P. indica confers freezing tolerance and better post-thaw recovery in Arabidopsis, and stimulates the expression of several genes involved in the CBF-dependent pathway.
Introduction
Freezing stress is an important threat limitating plant growth and yield,Citation1 and causes a negative effect on 15% of arable land around the world.Citation2 Freezing stress has been shown to affect membrane fluidity which is sensed by plasma membrane proteins, and calcium channels as well as their associated proteins. They activate calcium/calmodulin-dependent protein kinases (CPKs, CIPKs, and CRLK1) and the MAPK cascade, which is followed by the stimulation of CBF (C-repeat-binding factor) and COR (cold-regulated) gene expression.Citation3
Therefore, plants have developed sophisticated mechanisms in response to low-temperature stress, including alterations of the lipid composition, osmolyte accumulation like sugars and proline, and changes in the accumulation of protective proteins.Citation4 In Arabidopsis, many genes which respond to low-termperature stress have be described.Citation5,Citation6
The C-Repeat Binding Factor/Dehydration-Responsive-Element-Binding protein (CBF/DREB)-activated transcriptional regulatory cascade is one of the most important routes that triggers cold acclimation and the freezing tolerance pathway.Citation7,Citation8 The INDUCER OF CBF EXPRESSION 1 (ICE1), a basic helix-loop-helix (bHLH) transcription factor, directly binds and activates the expression of CBF/DREB1 to regulate the cold-response pathway in A. thaliana. The ICE-CBF-COR pathway has been widely studied over the past two decadesCitation7 and many regulators upstream and downstream of CBFs have been identified. MYB15,Citation9 EIN3,Citation10 ZAT12Citation11 and PIF3Citation12 function as negative regulators of the CBF pathway, while CAMATA3,Citation13 ERF105Citation14,Citation15 and BZR1Citation16 are considered as positive regulators. Freezing stress rapidly induces the expression of CBF genes, and the encoded proteins directly modulate a set of COR genes, which enhance freezing tolerance.Citation6
Arbuscular mycorrhizal symbiosis is well known to improve cold resistance in plants,Citation17–Citation20 and alleviates low-temperature stress in rice and barely.Citation21,Citation22 Also the root endophytic fungus Piriformospora indica, originally found in the cold Thar desert,Citation23 confers cold stress tolerance in its natural habitat and the cold desert of Leh-Ladakh.Citation24–Citation26 P. indica is well known for its stimulating effects on plant growth and survival under various abiotic stress conditions,Citation27 such as drought,Citation28–Citation30 saltCitation31-Citation33 and heavy metal stress.Citation34 Many reports showed that colonization of roots with P. indica activates the antioxidant defense system against salinity.Citation35 Phytohormones play important roles in the interaction between P. indica and plantsCitation36 including cold stress responses.Citation37 However, very little is known about the effects of this fungus on plant cold tolerance.Citation25,Citation38 Murphy et al.Citation38 showed that the fungus increases the yield in barley grown at low temperature. Up to date, no reports investigated the interaction between P. indica and Arabidopsis under cold stress. Since the mechanisms how P. indica influences freezing tolerance is only poorly understood, we used physiological and molecular parameters to investigate the effect of root colonization on the performance of Arabidopsis under freezing conditions.
Materials and methods
Plant materials and growth conditions
Three Arabidopsis thaliana ecotypes, Columbia (Col), Cape Verdi Island (Cvi-0), and C24 were used for the freezing experiments. The cbf1, cbf2, cbf3, cor47, cor15, siz1 and ice1 mutant plants were obtained from the Arabidopsis Biological Resource Center. Seeds of Arabidopsis were kept at 4°C for 3 days in dark for vernalization and then transferred to a growth chamber. After 7 days, seedlings were transferred to soil (peat: vermiculite: perlite; 3:1:1) with or without P. indica. The growth condition was 22°C with a 16-h-light/8-h-dark photoperiod.
P. indica propagation and inocculation assay
P. indica was propagated on PDA medium at 28°C in dark for a week. For liquid culture, 1000 ml Erlenmeyer flasks were filled with 200 ml liquid Aspergillus (ASP) medium and supplied with 5 fungal plugs before incubation for 10 days at 28°C at 130 rpm on a rotary shaker. For soil experiment, P. indica was cultured in ASP liquid medium for 10 days, then 200 µl of an 1% P. indica suspension was added to the soil in vicinity to the roots.Citation39
Root colonization analysis was performed according to a protocol modified from Nanda et al.Citation34 To confirm root colonization, 13 days post inoculation, roots of colonized Arabidopsis were washed with distilled water to remove medium and soil, then cut into 2.0 cm pieces, boiled for 1 h in 10% NaOH solution and incubated in 1% HCl for 10 min. Subsequently, roots were stained with 0.05% trypan blue overnight. Stained root segments were monitored under a light microscope at 20× magnification (Nikon CX41-72C02).
Freezing assays
Arabidopsis seedlings of the three ecotypes (Col, C24, Cvi-0) were grown at 22°C on soil for 15 days; subsequently they were exposed to the freezing treatment according to Hu et al.Citation40 in a freezing chamber (Panasonic, MIR-254-PC, Japan). Seedlings were maintained at 0°C for 1 h, then the temperature dropped by 2°C per hour to −6°C, where it was maintained for 6 h.Citation41 After the freezing treatment, seedlings were shifted to 4°C and kept in darkness for 12 h to thaw before the were transferred back to 22°C for 7 days (16-h-light/8-h-dark photoperiod). After recovery, the survival rates were calculated by counting the number of seedlings that produced new leaves.
Electrolyte leakage and water soaked area
After freezing, seedlings were collected in 10 ml tubes containing 5 ml of de-ionized water and the electrical conductivity (EC) was measured (S0). The samples were gently shaken at 22°C, and the EC was measured after 30 min (S1). Then, the samples were boiled for 15 min and shaken at 22°C, the EC was measured after additional 50 min (S2). Electrolyte leakage was calculated as follows: (S1 − S0)/(S2 − S0).Citation42 The water soaked area as determined by Image J as previously describedCitation43 and expressed as % of total aera.
Evaluation of freezing resistance of Arabidopsis leaves
Different paramenters were selected to evaluate freezing tolerance of Arabidopsis leaves. Soluble protein, proline, malondialdehyde (MDA) and ascorbic acid contents were determined using UV spectrophotometry (UV1800) according to Peng et al.Citation44 In addition, chlorophyll fluorescence parameters (Fv/Fm, NPQ, qp) were measured using a Chl fluorometer (Junior-PAM, Walz, Germany).Citation45 Water potential was detected by the PSYPRO water potential system (Wescor, Logan, UT, USA) as described in Wang and Shang.Citation46 Furthermore, nitro-blue tetrazolium (NBT) staining was used to detect superoxides (O2−), and diaminobezidin (DAB) staining to detect hydrogen peroxide (H2O2) as described by Huang et al.Citation1
Infrared thermal imaging
The leaf temperature was detected using an infrared camera. The thermal imaging experiments were carried out following the procedure described by Wang et al.Citation47 with slight modifications. Control plants and those after the freezing treatment were placed on the table and observed under an infrared camera with a temperature resolution as 0.05°C (IRBIS plus 3, InfraTec, Vario CAM® HiRes Germany). The infrared camera was installed vertically at approximately 30 cm above the leaf canopy for observation. Thermal images were analyzed subsequently through the IRBIS plus 3 software.
Quantification of endogenous hormones
The amounts of ethylene (ETH), jasmonate (JA), ABA and BR were measured in 15 d-old soil-grown plants after exposure to −6°C for 6 h. For the post-thaw recovery experiments (4°C), randomly selected leaves were harvested after 0, 3, 6, 12 and 24 h, and the hormone quantification occurred with an ELISA assay using monoclonal antibodies.Citation40
RNA isolation and quantitative PCR analysis
Total RNA was extracted from leaves of seedlings after exposure to the cooling gradient or during post-thaw recovery, using TRIzol. After reverse-transcription with the Reagent kit (Takara), quantitative real-time PCR was performed on the BIO-RAD CFX Connect Real-Time PCR system with SYBR Premix (ABI). The amounts of the amplification products were calculated as described.Citation10 ACTIN2 was used as an internal control. The primers are listed in Table S1.
Statistical analysis
Data display means with standard errors of three independent biological repeats. ANOVA was used and means were conducted by the Duncan’s multiple range tests (SPSS 14.0).
Results
P. indica enhances survival rate and ion leakage of freezing Arabidopsis
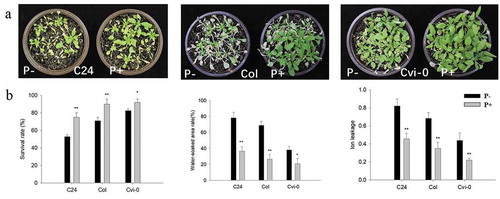
To examine the effect of P. indica on freezing tolerance in Arabidopsis, one-week-old seedlings were transferred to soil and co-cultivated with fresh mycelia of P. indica for two weeks (Figure S1). Although the three Arabidopsis ecotypes showed a quite different phenotype after freezing, the effect of the fungus was comparable (). The survival rates of the colonized seedlings (C24, 75%; Col, 89%; Cvi-0, 91%) was significantly higher than those of the uncolonized seedlings (C24, 52%; Col, 71%; Cvi-0, 83%) (). Notably, the areas which were soaked with water (expressed as % of total leaf areas) were significantly smaller and the electrolyte leakage indices significantly lower for colonized seedlings of the three ecotypes compared to the uncolonized controls (). It appears that the fungus plays an important role in reducing the ion leakage to restrict cell damage during freezing in the three investigated ecotypes.
Figure 1. Freezing phenotypes (a), survival rates (after 23 d on soil), water-soaked area (after 17 d on soil) and ion leakage (after 16 d on soil) (b) of C24, Col and Cvi-0 Arabidopsis seedlings. In (b), data are means of three independent experiments ± SD. Asterisks indicate significant differences compared with the non-inoculated plants (*P < .05, **P < .01, t-test). Black (white) bars: P. indica-(un-)colonized plants.
P. indica enhances antioxidants and osmolytes of Arabidopsis upon freezing stress
Because the effects of P. indica were comparable for the three ecotpyes, we focused on the Col ectotype for the following experiments. NBT staining of the leaves after the freezing treatment showed high superoxide (O2−) levels in colonized seedlings compared to the uncolonized controls (,c). On the other hand, DAB staining showed that the leaves of the colonized seedlings contained lower hydrogen peroxide (H2O2) levels (). Apparently, the O2− – generating damaged plastids are not protected by the fungus, while the the amount of ROS which preferentially accumulate in the apoplast under stress, is reduced by the fungus. Moreover, the total amount of ascorbic acid was stimulated by the fungus, while the amount of MDA, one of the final products of polyunsaturated fatty acids peroxidation in the cells, was lower in the leaves of colonized seedlings (). Apparently, the fungus allows a more efficient ROS scavenging.
Figure 2. Representative pictures of leaves after NBT (a) or DAB (b) staining. (c-g) quantified data for % area of leaves stained by NBT (c), and the amounts of soluble protein (d), proline (e), ascorbic acid (f), and MDA (g) in the leaves of wild-type seedlings after 12 h of post-thaw recovery period. Quantified data are means of three independent experiments ± SD. Asterisks indicate significant differences compared with the non-inoculated plants (*P < .05, **P < .01, t-test). Black (white) bars: P. indica-(un-)colonized plants.
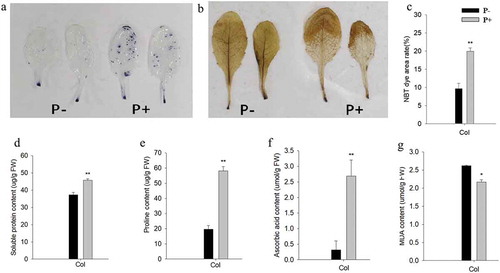
Besides stabilizing the membrane structures, osmolytes also play pivotal roles in almost all parts of the plant cells under freezing stress. demonstrates that the proline level, which is a critical marker freezing stress, is substantially increased in the leaves of colonized seedlings compared those of the uncolonized controls. This suggests that the inoculated plants accumulated more osmolytes, which may contribute to the freezing tolerance.
Leaf temperature, water potential and chlorophyll fluorescence parameters
The average leaf temperature of five randomly selected leaves from the different plants which were colonized by P. indica was 24.19°C and 23.94°C before and after freezing treatment, respectively (). The temperature in the leaves of the uncolonized plants was lower after the freezing stress (). Also, the leaf temperature of the colonized plants after freezing was comparable to that of the not cold-treated plants, while non-inoculated plants showed lower leaf temperature ().
Figure 3. Leaf temperature (a and b), water potential characteristics (c), chlorophyll fluorescence parameters (d, Fv/Fm; e,qp; f, NPQ) of Arabidopsis under freezing stress (−6°C). In b and d – f, data are means of three independent experiments ± SD. Asterisks indicate significant differences compared with the non-inoculated plants (*P < .05, **P < .01, t-test).
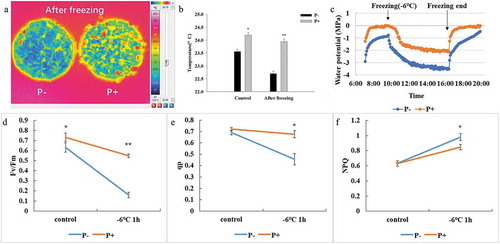
We also measured the water potentials and chlorophyll fluorescence parameters of Arabidopsis leaves under freezing stress. The water potential in the leaves of colonized seedlings was higher than that of the uncolonized controls (). In particular, after freezing, the water potential values in the colonized plants returned much faster to their original levels when compaired to the uncolonized controls. Furthermore, the Fv/Fm values and those for photochemical quenching (qp) decreased much faster in uncolonized seedlings after the freezing treatment (p < .05, ,e), while the non-photochemical quenching (NPQ) increased (p < .05, ). The fluorescence results demonstrate that the photosynthetic machinery in the colonized seedlings performs better during the freezing stress and recovers faster from the stress. The data are further supported by the water potential measurements, which are coupled to the photosynthetic parameters.
Effect of P. indica on hormone levels and hormone-regulated gene expression after recovery from freezing stress
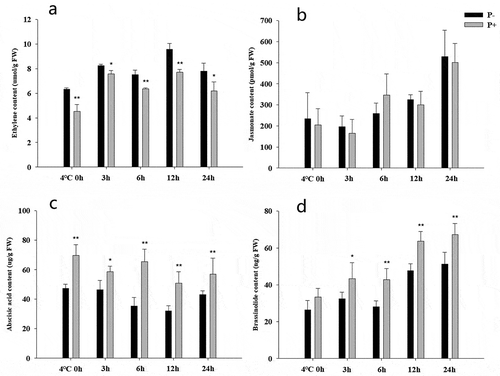
The phytohormone levels were measured during the first 24 h of the post-thaw recovery period. At all time points, colonized seedlings contained lower ETH levels (), whereas the ABA and BR levels were higher (,d). In contrast, no significant difference was observed for the JA levels (). These results indicate that P. indica increases freezing tolerance by manipulating the ETH, ABA and BR levels, while JA, predominantly involved in defense against biotic stress, is not influenced.
Figure 4. Phytohormone levels in Arabidopsis seedlings during the post-thaw recovery period. Ethylene (a), jasmonates (b), abscisic acid (c), brassinolide (d). Data are means of three independent experiments ± SD. Asterisks indicate significant differences compared to non-inoculated plants (*P < .05, **P < .01, t-test). Black (white) bars: P. indica-(un-)colonized plants.
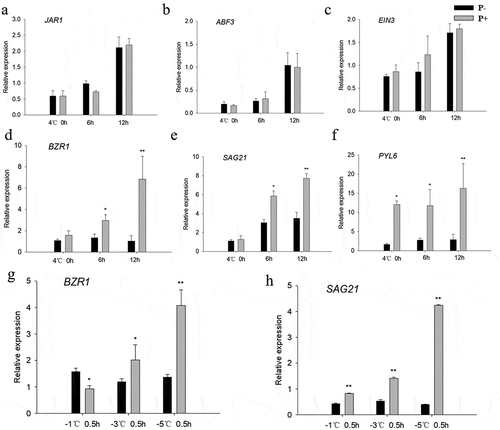
During the cooling periods () and post-thaw recovery (–), the BZR1 transcript level and the levels of the regulon members SAG21 and PYL6 were higher in Arabidopsis plants co-cultivated with P. indica compared to the uncolonized controls. No significant differences were found for other phytohormone-related genes (JAR1, ABF3, EIN3) (p > .05, –). These results show that P. indica increases the freezing tolerance by stimulating BR-regulated genes.
Figure 5. Relative mRNA levels of the phytohormone-related JAR1 (a), ABF3 (b), EIN3 (c), and the BR-related BZR1 (d), SAG21 (e) and PYL6 (f) genes in Columbia wild-type seedlings under cold stress. (g – h) BZR1 and SAG21 mRNA levels during the post-thaw recovery period. The values for non-inoculated wild-type (Col) plants was set to 1.00, and the values of colonized plants expressed relative to them. Data are means of three independent experiments ± SD. Asterisks indicate significant differences compared with the non-inoculated plants (*P < .05, **P < .01, t-test). Black (white) bars: P. indica-(un-)colonized plants.
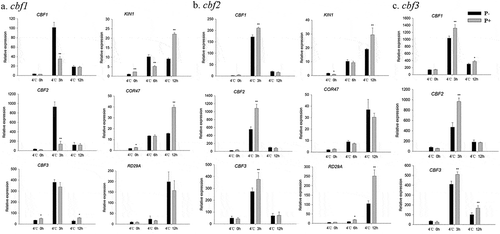
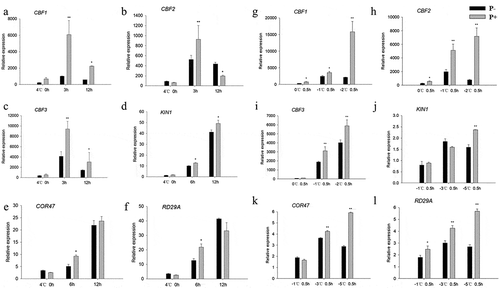
Response of CBF genes under freezing stress
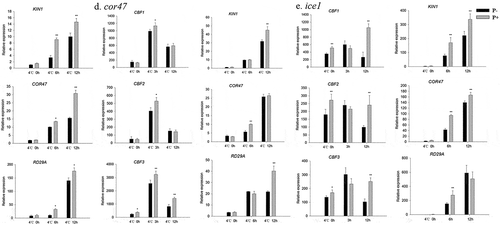
Since CBFs and their regulons (KIN1, COR47, RD29A) act as key transcription factors in the freezing signaling pathway,Citation6 we examined whether P. indica affects CBF gene expression during freezing in Arabidopsis. Compared to none-inoculated plants, the transcript levels for CBF1, −2 and −3 were higher in P. indica-inoculated plants, both during the cooling and the post-thaw recovery periods (– and –). Furthermore, also the investigated COR genes were higher expressed in colonized plants. These results suggest that the P. indica-induced freezing tolerance is associated with increased CBF and COR gene expression.
Figure 6. Relative mRNA levels of CBF and COR genes under cold stress and post-thaw recovery period. The values for non-inoculated wild-type (Col) plants was set to 1.00, and the values of the colonized plants expressed relative to them. Post-thaw recovery period (a – f), gradient cooling (g – l). Data are means of three independent experiments ± SD. Asterisks indicate significant differences compared with the non-inoculated plants (*P < .05, **P < .01, t-test). Black (white) bars: P. indica-(un-)colonized plants.
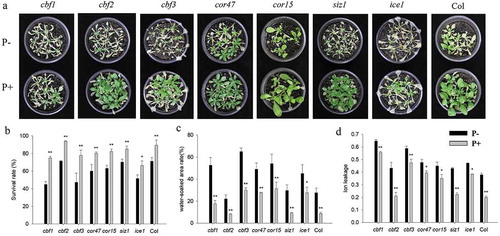
Mutants phenotyping and expression of freezing tolerance genes
In view of the central roles of the CBF and COR genes in regulating plant freezing tolerance, the related mutants (cbf1, cbf2, cbf3, cor47 and ice1) were subjected to the same freezing treatments as described for the wild-type. In all instances, the colonized mutant seedings showed high survival rates, and lower values for water-soaked areas and ion leakage (Figure 7a–d). We also examined the expression of CBF and COR genes in the cbf1, cbf2, cbf3, cor47 and ice1 mutants during the recovery period from freezing stress. The RT-qPCR analyzes show that the freezing treatment dramatically repressed the expression of CBFs at 4°C after 3 h. After 12 h, we observed a strong increase in the expression of the CBF genes in the inoculated cbf1 mutant (). The fungus also increased the expression of CBF and COR genes in the cbf2, cbf3, cor47 and ice1 mutants after the freezing stress (–). Interestingly, the responses to the fungus (i.e. the survival rate and stimulation of the expression of the investigated genes) were lower in the ice1 mutant when compared to those of the other mutants (Figures 7b, 8e), although a stimulatory effect was still detectable. These results indicated that ICE1 and CBF1 play prominent roles in the freezing tolerance response induced by P. indica, and the fungus targets components of the CBF-dependent pathway.
Discussion
ROS and ROS scavenging during recovery from freezing
Low and below 0°C temperatures restrict growth and productivity of plants worldwide,Citation20 mainly due to cell and tissue damage, and this is associated with the accumulation of ROS in the damaged tissues. Plants try to counteract this threat and evolved complex mechanisms to prevent damage caused by excess ROS formation under various environmental stresses.Citation48 Huang et al.Citation1 demonstrated that ROS scavenging enzymes and antioxidants accumulate during post-thaw recovery to restrict excess ROS accumulation. We demonstrate that P. indica-stimulated tolerance to freezing stress is associated with a signifcant increase in the ascorbic acid level, which likely participates in the reduction of the H2O2 content during the post-thaw recovery period. The lower MDA level in these plants confirms that colonized plants suffer less from the freezing stress (). Likewise, arbuscular mycorrhizal fungi have been shown to promote freezing tolerance by decreasing MDA levels in their hosts.Citation20–Citation22 Since freezing damages the integrity of cell membranes,Citation48 lower ROS levels in P. indica-colonized plants may restrict membrane peroxidation which may result in more efficient and faster recovery. The fungus also promotes the accumulation of osmotic substances during the post-thaw recovery period which might contribute to the tolerance response. How the plant immune system is strengthened during the recovery phase it unknown at present. Besides more general effects targeting the plant metabolism, it is worth testing whether specific proteins or metabolites which are secreted by the fungus in the symbiotic interactionCitation49,Citation50 participate in the freezing tolerance response. This is particular important for agriculture since many crops are subject to frost damage.Citation2
Figure 7. Freezing phenotypes (−6°C 6 h, a), survival rates (b), water-soaked area rate (c), ion leakage (d) of cbf1, cbf2, cbf3, cor47, cor15, siz1 and ice1 mutants. In b – d, data are means of three independent experiments ± SD. Asterisks indicate significant differences compared with the non-inoculated plants (*P < .05, **P < .01, t-test). Black (white) bars: P. indica-(un-)colonized plants.
Phytohormones and signaling in post-thaw recovery
Phytohormones are crucial players in cold stress responses.Citation37 We found that P. indica colonization alters the ABA, ETH and BR levels during the post-thaw recovery period, and inoculated plants produced more ABA and less ETH than non-inoculated plants (). This is consistant with previous reports that ABA positively and ETH negatively regulates freezing tolerance.Citation10,Citation42 It is long known that cold-responsive genes are regulated through C-repeat/dehydration-responsive and ABA-responsive cis-elements which are activated by binding to transacting and cold-regulated transcription factors.Citation51 An effect of P. indica on the ABA level in roots and shoots of different host plants have been repeatedly demonstrated (summarized in Xu et al.Citation36), however the contribtionof the hormone to freezing tolerance has not been investiated. A strong increase was reported during the early recognition phase and before a physical contact between the two symbiotic partners is established, suggesting that the ABA response in the host might be elicited by secreted fungal factors. Nummerous reports showed that also ETH plays an important role in the low temperature response of plants by controlling the CBF-dependent signaling pathway. However, the role of ETH in the freezing response is controversial. Shi et al.Citation10 showed that ETH has a negative effect on the freezing response in A. thaliana. Freezing tolerance was decreased in an ETH overproducer line and by the application of the ETH precursor 1-aminocyclopropane-1-carboxylic acid but increased by the addition of the ETH biosynthesis inhibitor aminoethoxyvinyl glycine or the perception antagonist Ag+. Furthermore, ETH-insensitive mutants, such as etr1-1, ein4-1, ein2-5, ein3-1, and ein3 eil1, displayed enhanced freezing tolerance. By contrast, the constitutive ETH response mutant ctr1-1 and EIN3-overexpressors exhibited reduced freezing tolerance. The authors showed that EIN3 negatively regulates the expression of CBFs and type-A RESPONSE REGULATOR5 (ARR5), ARR7, and ARR15 by binding to specific elements in their promoters. Thus, the detailed analyses of Shi et al.Citation10 demonstrated that ETH negatively regulates cold signaling at least partially through the direct transcriptional control of cold-regulated CBFs and type-A ARR genes by EIN3. Likewise, Zhao et al.Citation52 showed that cold acclimation-induced freezing tolerance of Medicago truncatula seedlings is negatively regulated by ETH. These results are consistent with our observations that P. indica decreases ETH accumulation during the post-thow period to promote the freezing tolerance response. On the other hand, Catalá and SalinasCitation53 showed that the Arabidopsis ETH overproducer eto1-3 displays enhanced freezing tolerance and Popov et al.Citation54 reported that the ETH-insensitive Arabidopsis mutants etr1-1 and ein2-1 have a decreased freezing tolerance. The quite different experimental set-ups used for these experiments might be responsible for the different results.
The JA content was not significantly different between non-inoculated and inoculated Arabidopsis under freezing stress (). Thus, our JA results provides a control for a hormone that is known to be involved in biotic stress responses.
BR signaling regulates a variety of stress responses, however its involvement in freezing responses is not well studied. Li et al.Citation16 showed that the BR-controlled transcription factor CESTA plays a key role in BR-improved Arabidopsis freezing tolerance. Our results showed that the BR-related transcript levels for BZR1, SAG1 and PYL6 were up-regulated during freezing stress and post-thaw recovery, and this effect was further stimulated by P. indica (, ). The results suggest that BR signaling compounds are targets of the fungus in the freezing tolerance response.
Taken together, our results suggest that inoculation of P. indica increases plant resistance to freezing stress by increasing the ABA and BR levels. Furthermore, higher BR levels and transcript levels of BR-targeted genes may improve post-thaw recovery in the presence of the fungus.
Figure 8. Relative mRNA levels of CBF and COR genes in the cbf1 (a), cbf2 (b), cbf3 (c), cor47 (d) and ice1 (e) mutants during the post-thaw recovery period. The values for non-inoculated wild-type (Col) plants was set to 1.00, and the values of the colonized plants expressed relative to them. Data are means of three independent experiments ± SD. Asterisks indicate significant differences compared to the non-inoculated plants (*P < .05, **P < .01, t-test). Black (white) bars: P. indica-(un-)colonized plants.
P. indica increases freezing resistance of Arabidopsis by stimulating CBF and COR genes
The CBF pathway plays a critical role in plant freezing tolerance, and it is of vital important to understand the mechanisms that control this signaling pathway.Citation42 Our results show that the CBF and COR genes were induced under freezing conditions, and their expression was further stimulated by P. indica, both under freezing stress and during the post-thaw recovery (), as well as in mutants impaired in freezing tolerance-related responses. This suggests that P. indica activates the ICE-CBF-COR pathway to enhance freezing tolerance in Arabidopsis. However, since P. indica still stimulates freezing tolerance in various mutants impaired in components of the CBF pathway (), CBF-independent targets of the fungus may also be involved in the P. indica effect. Alternatively, investigation of single mutants may not be enough to inhibit the beneficial effect of the fungus. The P. indica enhance on mutant plants, possibly due to the effect of gene member redundance, for example, CBF1, 2, 3 have synergistic effects. Apparently, the fungus does not activate just one several gene/protein involved in the freezing tolerance. For example, P. indica stimulated the BR level which resulted also in the activation of CBF-independent COR genes ( and ).Citation16 The stimulatory effect of P. indica on the CBF transcript level reaches its maximum 3 h after the freezing period during post-thaw recovery (). A possible scenario could be that P. indica regulates CBF expression by activating the BR transcription factor genes BZR1, SAG1 and PYL6 during early stages of freezing stress. The complexity of the regulatory curcuits requires more detailed analyses.
Previous reports demonstrated that mutants (except cbf2) used in our study displayed freezing-sensitive phenotypes.Citation7,Citation41,Citation42 Our results are consistent with these reports (). Surprisingly, the inoculated ice1 mutant showed a lower increase in the survival rate and gene expression level compared to the wild-type (). ICE1 activates CBF3 expressionCitation42 and CBF3 expression responds to the fungus even in the ice1 background (Figure 8e). This suggests that P. indica functions downstram of ICE1. Furthermore, P. indica enhances freezing tolerance in the single cbf mutants (), and activates their COR genes (–c). The fungus also confers freezing tolerance to the cor47, cor15 and siz1 mutants (). These results argue in favor of multiple targets of the fungus in the freezing tolerance pathway or a high redundancy of the gene products ().
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download MS Word (1 MB)Acknowledgments
We acknowledge Xiaobing Feng for her contribution in data collection, Sebastian Buitrago and Rong Zeng for valuable discussion on the original draft. We also thank Qiao Wei for his technical support.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Huang X, Zhang Y, Zhang X, Shi Y. Long-chain base kinase1 affects freezing tolerance in Arabidopsis thaliana. Plant Sci. 2017;259:1–11. doi:10.1016/j.plantsci.2017.03.009.
- Nah G, Lee M, Kim DS, Rayburn AL, Voigt T, Lee DK. Transcriptome analysis of Spartina pectinata in response to freezing stress. PLoS One. 2016;11:e0152294. doi:10.1371/journal.pone.0152294.
- Zhu JK. Abiotic stress signaling and responses in plants. Cell. 2016;167:313–324. doi:10.1016/j.cell.2016.08.029.
- Bredow M, Vanderbeld B, Walker VK. Ice-binding proteins confer freezing tolerance in transgenic Arabidopsis thaliana. Plant Biotechnol J. 2017;15:68–81. doi:10.1111/pbi.12592.
- Guo X, Liu D, Chong K. Cold signaling in plants: insights into mechanisms and regulation. J Integr Plant Biol. 2018;60:745–756. doi:10.1111/jipb.12706.
- Ding Y, Shi Y, Yang S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019;222:1690–1704. doi:10.1111/nph.15696.
- Shi Y, Ding Y, Yang S. Molecular regulation of CBF signaling in cold acclimation. Trends Plant Sci. 2018;23:623–637. doi:10.1016/j.tplants.2018.04.002.
- Ren Y, Miao M, Meng Y, Cao J, Fan T, Yue J, Xiao F, Liu Y, Cao S. DFR1-mediated inhibition of proline degradation pathway regulates drought and freezing tolerance in Arabidopsis. Cell Rep. 2018;23:3960–3974. doi:10.1016/j.celrep.2018.04.011.
- Kim SH, Kim HS, Bahk S, An J, Yoo Y, Kim JY, Chung WS. Phosphorylation of the transcriptional repressor MYB15 by mitogen-activated protein kinase 6 is required for freezing tolerance in Arabidopsis. Nucl Acid Res. 2017;45:6613–6627. doi:10.1093/nar/gkx417.
- Shi Y, Tian S, Hou L, Huang X, Zhang X, Guo H, Yang S. Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. Plant Cell. 2012;24:2578–2595. doi:10.1105/tpc.112.098640.
- Vogel JT, Zarka DG, Buskirk HAV, Fowler SG, Thomashow MF. Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J. 2010;41:195–211. doi:10.1111/j.1365-313X.2004.02288.x.
- Jiang B, Shi Y, Zhang X, Xin X, Qi L, Guo H, Li J, Yang S. PIF3 is a negative regulator of the CBF pathway and freezing tolerance in Arabidopsis. Proc Natl Acad Sci. 2017;114:E6695–E6702. doi:10.1073/pnas.1706226114.
- Doherty CJ, Buskirk HAV, Myers SJ, Thomashow MF. Roles for Arabidopsis CAMTA transcription factors in cold-regulated gene expression and freezing tolerance. Plant Cell. 2009;21:972–984. doi:10.1105/tpc.108.063958.
- Bolt S, Zuther E, Zintl S, Hincha DK, Schmülling T. ERF105 is a transcription factor gene of Arabidopsisthaliana required for freezing tolerance and cold acclimation. Plant Cell Environ. 2017;40:108–120. doi:10.1111/pce.12838.
- Cai W, Yang Y, Wang W, Guo G, Liu W, Bi C. Overexpression of a wheat (Triticum aestivum L.) bZIP transcription factor gene, TabZIP6, decreased the freezing tolerance of transgenic Arabidopsis seedlings by down-regulating the expression of CBFs. Plant Physiol Biochem. 2018;124:100–111. doi:10.1016/j.plaphy.2018.01.008.
- Li H, Ye K, Shi Y, Cheng J, Zhang X, Yang S. BZR1 positively regulates freezing tolerance via CBF-dependent and CBF-independent pathways in Arabidopsis. Mol Plant. 2017;10:545–559. doi:10.1016/j.molp.2017.01.004.
- Aroca R, Porcel R, Ruiz‐Lozano JM. How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytol. 2007;173:808–816. doi:10.1111/j.1469-8137.2006.01961.x.
- Ma J, Janoušková M, Li Y, Yu X, Yan Y, Zou Z, He C. Impact of arbuscular mycorrhizal fungi (AMF) on cucumber growth and phosphorus uptake under cold stress. Funct Plant Biol. 2015;42:1158–1167. doi:doi.10.1071/FP15106.
- Chu XT, Fu JJ, Sun YF, Xu YM, Miao YJ, Xu YF, Hu TM. Effect of arbuscular mycorrhizal fungi inoculation on cold stress-induced oxidative damage in leaves of Elymus nutans Griseb. S Afr J Bot. 2016;104:21–29. doi:10.1016/j.sajb.2015.10.001.
- Zhu X, Song F, Liu F. Arbuscular mycorrhizal fungi and tolerance of temperature stress in plants. In: Wu QS, editor. Arbuscular mycorrhizas and stress tolerance of plants. Singapore: Springer. 2017;p. 163–194. doi:10.1007/978-981-10-4115-0_8.
- Liu Z, Li Y, Wang J, He X. Different respiration metabolism between mycorrhizal and non-mycorrhizal rice under low-temperature stress: a cry for help from the host. J Agr Sci. 2015;153:602–614. doi:10.1017/S0021859614000434.
- Hajiboland R, Joudmand A, Aliasgharzad N, Tolrá R, Poschenrieder C. Arbuscular mycorrhizal fungi alleviate low-temperature stress and increase freezing resistance as a substitute for acclimation treatment in barley. Crop Pas Sci. 2019;70:218–233. doi:doi.10.1071/CP18385.
- Ye W, Jiang J, Lin Y, Yeh K, Lai Z, Xu X, Oelmüller R. Colonisation of Oncidium orchid roots by the endophyte Piriformospora indica restricts Erwinia chrysanthemi infection, stimulates accumulation of NBS-LRR resistance gene transcripts and represses their targeting micro-RNAs in leaves. BMC Plant Biol. 2019;19:1–16. doi:10.1186/s12870-019-2105-3.
- Varma A, Bakshi M, Lou B, Hartmann A, Oelmüller R. Piriformospora indica: a novel plant growth-promoting mycorrhizal fungus. Agric Res. 2012;1:117–131. doi:10.1007/s40003-012-0019-5.
- Li D, Mensah RA, Liu F, Tian N, Qi Q, Yeh K, Xu X, Cheng C, Lai Z. Effects of Piriformospora indica on rooting and growth of tissue-cultured banana (Musa acuminata cv. Tianbaojiao) seedlings. Sci Hortic. 2019;257:108649. doi:10.1016/j.scienta.2019.108649.
- Varma A, Sree KS, Arora M, Bajaj R, Prasad R, Kharkwal AC. Functions of novel symbiotic fungus-Piriformospora indica. Proc Indian Natl Sci Acad. 2014;80:429–441. doi:10.16943/ptinsa/2014/v80i2/55119.
- Lin HF, Xiong J, Zhou HM, Chen CM, Lin FZ, Xu XM, Oelmüller R, Xu WF, Yeh KW. Growth promotion and disease resistance induced in Anthurium colonized by the beneficial root endophyte Piriformospora indica. BMC Plant Biol. 2019;19:40. doi:10.1186/s12870-019-1649-6.
- Xu L, Wang A, Wang J, Wei Q, Zhang W. Piriformospora indica confers drought tolerance on Zea mays L. through enhancement of antioxidant activity and expression of drought-related genes. Crop J. 2017;5:251–258. doi:10.1016/j.cj.2016.10.002.
- Saddique MAB, Ali Z, Khan AS, Rana IA, Shamsi IH. Inoculation with the endophyte Piriformospora indica significantly affects mechanisms involved in osmotic stress in rice. Rice. 2018;11:34. doi:10.1186/s12284-018-0226-1.
- Zhang W, Wang J, Xu L, Wang A, Huang L, Du H, Qiu L, Oelmüller R. Drought stress responses in maize are diminished by Piriformospora indica. Plant Signal Behav. 2018;13:e1414121. doi:10.1080/15592324.2017.1414121.
- Abdelaziz ME, Kim D, Ali S, Fedoroff NV, Al-Babili S. The endophytic fungus Piriformospora indica enhances Arabidopsis thaliana growth and modulates Na+/K+ homeostasis under salt stress conditions. Plant Sci. 2017;263:107–115. doi:10.1016/j.plantsci.2017.07.006.
- Abdelaziz ME, Abdelsattar M, Abdeldaym EA, Atia MAM, Mahmoud AWM, Saad MM, Hirt H. Piriformospora indica alters Na+/K+ homeostasis, antioxidant enzymes and LeNHX1 expression of greenhouse tomato grown under salt stress. Sci Hortic. 2019;256:108532. doi:10.1016/j.scienta.2019.05.059.
- Yun P, Xu L, Wang S, Lana S, Sergey S, Zhang W. Piriformospora indica improves salinity stress tolerance in Zea mays L. plants by regulating Na+ and K+ loading in root and allocating K+ in shoot. Plant Growth Regul. 2018;86:323–331. doi:10.1007/s10725-018-0431-3.
- Hui F, Liu J, Gao Q, Lou B. Piriformospora indica confers cadmium tolerance in Nicotiana tabacum. J Enviro Sci. 2015;37:184–191. doi:10.1016/j.jes.2015.06.005.
- Baltruschat H, Fodor J, Harrach BD, Niemczyk E, Barna B, Gullner G, Janeczko A, Kogel KH, Schäfer P, Schwarczinger I, et al. Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol. 2010;180:501–510. doi:10.1111/j.1469-8137.2008.02583.x.
- Xu L, Wu C, Oelmüller R, Zhang W. Role of phytohormones in Piriformospora indica-induced growth promotion and stress tolerance in plants: more questions than answers. Front Microbiol. 2018;9:1646. doi:10.3389/fmicb.2018.01646.
- Hu Y, Jiang Y, Han X, Wang H, Pan J, Yu D. Jasmonate regulates leaf senescence and tolerance to cold stress: crosstalk with other phytohormones. J Exp Bot. 2017;68:1361–1369. doi:10.1093/jxb/erx004.
- Murphy BR, Doohan FM, Hodkinson TR. Yield increase induced by the fungal root endophyte Piriformospora indica in barley grown at low temperature is nutrient limited. Symbiosis. 2014;62:29–39. doi:10.1007/s13199-014-0268-0.
- Pan R, Xu L, Wei Q, Wu C, Tang W, Oelmüller R, Zhang W. Piriformospora indica promotes early flowering in Arabidopsis through regulation of the photoperiod and gibberellin pathways. PLoS One. 2017;12:e0189791. doi:10.1371/journal.pone.0189791.
- Hu Y, Jiang L, Wang F, Yu D. Jasmonate regulates the inducer of CBF expression–c-repeat binding factor/DRE binding factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell. 2013;25:2907–2924. doi:10.1105/tpc.113.112631.
- Novillo F, Alonso JM, Ecker JR, Salinas J. CBF2/DREB1C is a negative regulator of CBF1/DREB1B and CBF3/DREB1A expression and plays a central role in stress tolerance in Arabidopsis. Proc Natl Acad Sci. 2004;101:3985–3990. doi:10.1073/pnas.0303029101.
- Ding Y, Li H, Zhang X, Xie Q, Gong Z, Yang S. OST1 kinase modulates freezing tolerance by enhancing ICE1 stability in Arabidopsis. Dev Cell. 2015;32:278–289. doi:10.1016/j.devcel.2014.12.023.
- Lukatkin AS, Brazaityte A, Bobinas C, Duchovskis P. Chilling injury in chilling-sensitive plants: a review. Agriculture. 2012;99:111–124. doi:10.3906/tar-1112-44.
- Peng L, Xu Y, Wang X, Feng X, Zhao QQ, Feng SS, Zhao ZY, Hu BZ, Li FL. Overexpression of paralogues of the wheat expansin gene TaEXPA 8 improves low‐temperature tolerance in Arabidopsis. Plant Biol. 2019;21:1119–1131. doi:10.1111/plb.13018.
- Pan R, Jiang W, Wang Q, Xu L, Shabala S, Zhang W. Differential response of growth and photosynthesis in diverse cotton genotypes under hypoxia stress. Photosynthetica. 2019;57:772–779. doi:10.32615/ps.2019.087.
- Wang H, Shang Q. Identification and functional analysis of proteins in response to light intensity, temperature and water potential in Brassica rapa hypocotyl. Physiol Plant. 2019;167:48–63. doi:10.1111/ppl.12865.
- Wang J, Guo Y, Ding B, Li X, Liu Y, Xie X. Screening of stomatal mutants inArabidopsisusing a novel controlled environmental infrared imaging system. Plant Growth Regul. 2016;79:157–165. doi:10.1007/s10725-015-0121-3.
- Arora R. Mechanism of freeze-thaw injury and recovery: A cool retrospective and warming up to new ideas. Plant Sci. 2018;270:301–313. doi:10.1016/j.plantsci.2018.03.002.
- Thürich J, Meichsner D, Furch ACU, Pfalz J, Krüger T, Kniemeyer O, Oeimüller R. Arabidopsis thaliana responds to colonisation of Piriformospora indica by secretion of symbiosis-specific proteins. PLoS One. 2018;13:e0209658. doi:10.1371/journal.pone.0209658.
- Liu L, Xu L, Jia Q, Pan R, Oeimüller R, Zhang W, Wu C. Arms race: diverse effector proteins with conserved motifs. Plant Signal Behav. 2019;14:1557008. doi:10.1080/15592324.2018.1557008.
- Viswanathan C, Zhu JK. Molecular genetic analysis of cold–regulated gene transcription. Philos Trans R Soc Lond B Biol Sci. 2002;357:877–886. doi:10.1098/rstb.2002.1076.
- Zhao M, Liu W, Xia X, Wang T, Zhang WH. Cold acclimation‐induced freezing tolerance of Medicago truncatula seedlings is negatively regulated by ethylene. Physiol Plant. 2014;152:115–129. doi:10.1111/ppl.12161.
- Catalá R, Salinas J. The Arabidopsis ethylene overproducer mutant eto1-3 displays enhanced freezing tolerance. Plant Signal Behav. 2015;10:e989768. doi:10.4161/15592324.2014.989768.
- Popov VN, Deryabin AN, Astakhova NV, Antipina OV, Suvorova TA, Alieva GP, Moshkov IE. Ethylene-Insensitive Arabidopsis mutants etr1-1 and ein2-1 have a decreased freezing tolerance. Dokl Biochem Biophys. 2019;487:269–271. doi:10.1134/S1607672919040069.