ABSTRACT
Arabidopsis thaliana seedlings exhibit longer hypocotyls when they are grown under high ambient temperature, which is defined as thermomorphogenesis. Although it is well established that high temperature triggers auxin biosynthesis to stimulate hypocotyl elongation, the physiological functions of other endogenous phytohormones during thermomorphogenesis are still elusive. Here, we report that exogenous application of abscisic acid (ABA) strongly inhibits hypocotyl elongation under high ambient temperature. Hypocotyl elongations of ABA biosynthesis deficient mutants are more sensitive to high temperature, suggesting that endogenous ABA has a robust inhibition effect. Moreover, blocking ABA perception or signaling impedes the negative effect of ABA. Finally, we show that ABA also suppresses the hypersensitivity to high temperature of an auxin over-accumulation mutant (yuc1D), indicating that activation of auxin signaling is not sufficient to override the repression by ABA. Taken together, we demonstrate that ABA is a negative regulator during plant thermomorphogenesis.
As sessile organisms, plants cannot change their locations when they are facing unfavorable environmental conditions. For example, with the global warming, high ambient temperature is becoming an inevitable cue surrounding land plants. For adapting to high temperature, rosette leaves in the reference plants Arabidopsis thaliana form much longer petioles and grow up-wards. Light grown seedlings also show elongated hypocotyls under high temperature.Citation1 These architecture changes collectively termed thermomorphogenesis, which helps plants to cool down their leaf surface temperature.Citation2 Recent studies have shown that plant photoreceptor phytochromes act as thermo-sensors to sense the temperature change.Citation3,Citation4 High temperature inactivates phytochromes to release their repression on the transcription factor PHYTOCHROME INTERACTING FACTOR 4 (PIF4).Citation5 PIF4 directly up-regulates the expression levels of several auxin biosynthesis or signaling related genes to stimulate auxin signaling, which further induces cell elongation.Citation6–Citation8
High ambient temperature usually co-exist with drought due to water evaporation. Abscisic acid (ABA) is the most important phytohormone to modulate plant drought tolerance. After drought stress, ABA is rapidly de novo synthesized or de-conjugated from its inactive conjugated forms. ABA then binds to its receptors PYRABACTIN RESISTANCE 1 (PYR1) and PYR1-LIKE (PYL) proteins, which further interact with the clade A type 2 C protein phosphatases (PP2Cs) to release their repression on the SnRK2 protein kinases.Citation9–Citation11 SnRK2 protein kinases then phosphorylate their substrate transcription factors or ion channels to elicit various ABA responses, including growth inhibition and stomata closure.Citation12
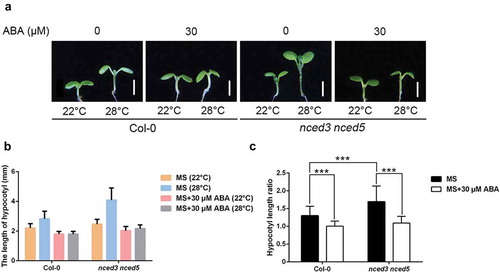
To test whether ABA is involved in thermomorphogenesis or not, we initially characterized hypocotyl elongation phenotypes in the wild-type (Col-0 ecotype) seedlings after different concentrations of ABA treatment. Without exogenous ABA treatment, hypocotyls elongated under 28°C. However, in the presence of ABA, ABA strongly suppressed hypocotyl elongation in a dosage dependent manner (). Next, we took advantage of an ABA-deficient mutant to dissect the role of endogenous ABA during thermomorphogenesis. 9-cis-epoxycarotenoid dioxygenase (NCED) is a key enzyme for ABA biosynthesis. NCED3 and NCED5 are required for ABA accumulation in A. thaliana under normal growth condition.Citation13,Citation14 We found that nced3 nced5 double mutants were more sensitive to high temperature (,b)), while exogenous ABA supplement rescued their hypersensitivity (–c)). Taken together, these results indicate that no matter endogenous or exogenous ABA suppressed hypocotyl elongation during thermomorphogenesis.
Figure 1. ABA suppresses high temperature-induced hypocotyl elongation.
(a) Representative images showing hypocotyls of the wild-type (Col-0) grown on the MS medium supplemented with different concentrations of ABA at 22°C or 28°C. Scale bar = 2 mm. (b) Quantitative results showing hypocotyl lengths of Col-0 grown on the MS medium supplemented with different concentrations of ABA at 22°C or 28°C. Mean ± SD; n = 30.
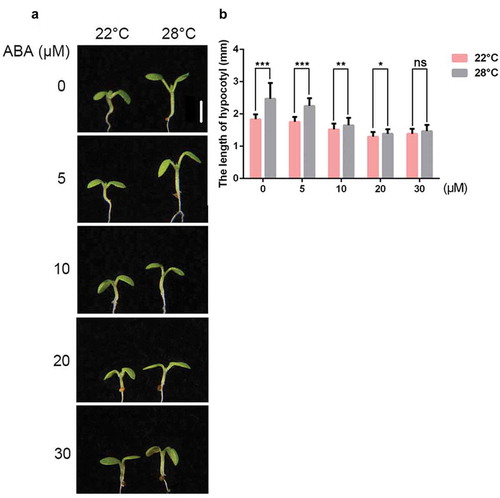
Figure 2. Hypocotyl length of nced3 nced5.
(a) Representative images showing hypocotyl length of Col-0 and nced3 nced5 double mutants grown in the presence of 30 μM ABA or not at 22°C or 28°C. Scale bar = 2 mm. (b, c) Quantitative results for hypocotyl length (b) and relative hypocotyl length ratio (28°C vs 22°C) (c) as shown in (a). Mean ± SD; n = 30.
Next, we asked if the core ABA signaling is required for suppressing thermomorphogenesis. Similar to Col-0, the sextuple ABA receptor mutants (pyr1 pyl1 pyl2 pyl4 pyl5 pyl8) exhibited elongated hypocotyls under 28°C under MS medium (–c)). ABA treatment completely suppressed high temperature–induced hypocotyl elongation in Col-0, however, pyr1 pyl1 pyl2 pyl4 pyl5 pyl8 mutants still displayed elongated hypocotyls (–c)). Due to functional redundancy with other PYL receptors,Citation15,Citation16 hypocotyls of pyr1 pyl1 pyl2 pyl4 pyl5 pyl8 mutants grown under ABA treatment at 28°C were slightly shorter than mock treatment ()). Nonetheless, we conclude that ABA receptors are required for suppressing thermomorphogenesis.
Figure 3. Hypocotyl length of core ABA signaling mutants.
(a) Representative images showing hypocotyl length of Col-0 and pyr1 pyl1 pyl2 pyl4 pyl5 pyl8 mutants grown in the presence of 30 μM ABA or not at 22°C or 28°C. Scale bar = 2 mm. (b,c) Quantitative results for hypocotyl length (b) and relative hypocotyl length ratio (28°C vs 22°C) (c) as shown in (a). Values shown are means ± SD; n = 30. (d) Representative images showing hypocotyl length of Ler and abi1-1mutants grown in the presence of 30 μM ABA or not at 22°C or 28°C. Scale bar = 2 mm. (e,f) Quantitative results for hypocotyl length (e) and relative hypocotyl length ratio (28°C vs 22°C) (f) as shown in (d). Means ± SD; n = 30.

ABA INSENSITIVE 1 (ABI1) is one of the key PP2Cs in the core ABA signaling.Citation17,Citation18 Dominant mutation of ABI1 (abi1-1) results in constitutive ABA insensitivity due to the dissociation with ABA receptors.Citation11 ABA suppressed hypocotyl elongation under high temperature in the background control (Ler), while the suppression was attenuated in the abi1-1 mutants (–f)).
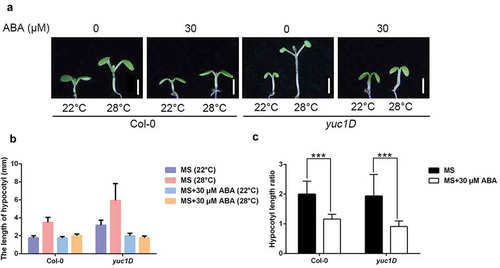
The above results illustrate that ABA inhibits thermomorphogenesis through the canonical ABA signaling. Finally, we tried to test if auxin over-accumulation could antagonize the negative effect of ABA. High temperature triggers auxin biosynthesis to boost cell elongation. Consistently, auxin over-accumulation mutants yuc1D displayed much longer hypocotyls under 28°C (,b)). However, ABA treatment successfully suppressed hypocotyl elongation in both Col-0 and yuc1D, suggesting that even activation of auxin signaling could not bypass the repression effect of ABA (–c)).
Figure 4. Hypocotyl length of yuc1D.
(a) Representative images showing hypocotyl length of Col-0 and yuc1D mutants grown in the presence of 30 μM ABA or not at 22°C or 28°C. Scale bar = 2 mm. (b, c) Quantitative results for hypocotyl length (b) and relative hypocotyl length ratio (28°C vs 22°C) (c) as shown in (a). Means ± SD; n = 30.
In summary, we report that ABA inhibits thermomorphogenesis in A. thaliana seedlings through the canonical ABA signaling. We also show that ABA suppresses the hypersensitivity to high temperature in auxin over-accumulation mutants.
High temperature and drought conditions are usually linked in natural. High temperature triggers cell elongation to escape unfavorable condition, while drought elicits ABA signaling to close stomata as a tolerance strategy. We assume that plants have to coordinate these two different mechanisms for better adapting to high temperature.
Materials and methods
Materials and growth conditions
Mutant seeds of nced3 nced5,Citation13 pyr1 pyl1 pyl2 pyl4 pyl5 pyl8,Citation15 abi1-1Citation17 and yuc1DCitation19 have been described previously. Seeds were sterilized with 10% sodium hypochlorite and 0.1% Triton X-100 for five minutes, rinsed five times with sterile water and then placed on Murashige and Skoog (MS) medium plates (4.4 g/L MS salt; 1.5% sucrose; pH 5.8; 1% agar). After stratification at 4°C for three days, seedlings were initially grown at 22°C for four days and then carefully transferred to MS medium or MS medium supplemented with different concentrations of ABA for another four days under 22°C or 28°C.
Phenotypic observations
Hypocotyls were imaged under a stereomicroscope and then measured using ImageJ software (http://rsbweb.nih.gov/ij/). Statistic significance was determined using multiple t test (*** P <.001; ** P <.01; * P <.05).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Yang Zhao for pyr1 pyl1 pyl2 pyl4 pyl5 pyl8 and nced3 nced5 seeds and The European Arabidopsis Stock Centre for abi1-1 seeds.
Additional information
Funding
References
- Quint M, Delker C, Franklin KA, Wigge PA, Halliday KJ, van Zanten M. Molecular and genetic control of plant thermomorphogenesis. Nat Plants. 2016;2:1. doi:10.1038/nplants.2015.190.
- Crawford AJ, McLachlan DH, Hetherington AM, Franklin KA. High temperature exposure increases plant cooling capacity. Curr Biol. 2012;22:R396–5. doi:10.1016/j.cub.2012.03.044.
- Jung JH, Domijan M, Klose C, Biswas S, Ezer D, Gao M, Khattak AK, Box MS, Charoensawan V, Cortijo S, et al. Phytochromes function as thermosensors in Arabidopsis. Science. 2016;354:886–889. doi:10.1126/science.aaf6005.
- Legris M, Klose C, Burgie ES, Rojas CC, Neme M, Hiltbrunner A, Wigge PA, Schafer E, Vierstra RD, Casal JJ. Phytochrome B integrates light and temperature signals in Arabidopsis. Science. 2016;354:897–900. doi:10.1126/science.aaf5656.
- Jin H, Zhu Z. Dark, light, and temperature: key players in plant morphogenesis. Plant Physiol. 2019;180:1793–1802. doi:10.1104/pp.19.00331.
- Franklin KA, Lee SH, Patel D, Kumar SV, Spartz AK, Gu C, Ye S, Yu P, Breen G, Cohen JD, et al. Phytochrome-interacting factor 4 (PIF4) regulates auxin biosynthesis at high temperature. Proc Natl Acad Sci U S A. 2011;108:20231–20235. doi:10.1073/pnas.1110682108.
- Koini MA, Alvey L, Allen T, Tilley CA, Harberd NP, Whitelam GC, Franklin KA. High temperature-mediated adaptations in plant architecture require the bHLH transcription factor PIF4. Curr Biol. 2009;19:408–413. doi:10.1016/j.cub.2009.01.046.
- Sun J, Qi L, Li Y, Chu J, Li C. PIF4-mediated activation of YUCCA8 expression integrates temperature into the auxin pathway in regulating arabidopsis hypocotyl growth. PLoS Genet. 2012;8:e1002594. doi:10.1371/journal.pgen.1002594.
- Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, Park SY, Cutler SR, Sheen J, Rodriguez PL, Zhu JK. In vitro reconstitution of an abscisic acid signalling pathway. Nature. 2009;462:660–664. doi:10.1038/nature08599.
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–1068. doi:10.1126/science.1172408.
- Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–1071. doi:10.1126/science.1173041.
- Chen K, Li GJ, Bressan RA, Song CP, Zhu JK, Zhao Y. Abscisic acid dynamics, signaling, and functions in plants. J Integr Plant Biol. 2020;62:25–54. doi:10.1111/jipb.12899.
- Frey A, Effroy D, Lefebvre V, Seo M, Perreau F, Berger A, Sechet J, To A, North HM, Marion-Poll A. Epoxycarotenoid cleavage by NCED5 fine-tunes ABA accumulation and affects seed dormancy and drought tolerance with other NCED family members. Plant J. 2012;70:501–512. doi:10.1111/j.1365-313X.2011.04887.x.
- Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001;27:325–333. doi:10.1046/j.1365-313x.2001.01096.x.
- Gonzalez-Guzman M, Pizzio GA, Antoni R, Vera-Sirera F, Merilo E, Bassel GW, Fernandez MA, Holdsworth MJ, Perez-Amador MA, Kollist H, et al. Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. Plant Cell. 2012;24:2483–2496. doi:10.1105/tpc.112.098574.
- Zhao Y, Zhang Z, Gao J, Wang P, Hu T, Wang Z, Hou YJ, Wan Y, Liu W, Xie S, et al. Arabidopsis duodecuple mutant of PYL ABA receptors reveals PYL repression of ABA-independent SnRK2 activity. Cell Rep. 2018;23:3340–3351 e3345. doi:10.1016/j.celrep.2018.05.044.
- Meyer K, Leube MP, Grill E. A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science. 1994;264:1452–1455. doi:10.1126/science.8197457.
- Wu Y, Sanchez JP, Lopez-Molina L, Himmelbach A, Grill E, Chua NH. The abi1-1 mutation blocks ABA signaling downstream of cADPR action. Plant J. 2003;34:307–315. doi:10.1046/j.1365-313X.2003.01721.x.
- Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science. 2001;291:306–309. doi:10.1126/science.291.5502.306.
