ABSTRACT
Calcium (Ca2+) as a universal signal molecule plays pivotal roles in plant growth and development. It regulates root morphogenesis mainly through mediating phytohormone and stress signalings or affecting these signalings. In recent years, much progress has been made in understanding the roles of Ca2+ in primary root development. Here, we summarize recent advances in the functions and mechanisms of Ca2+ in modulating primary root growth in plants under normal and stressful conditions.
Text
Root system including the primary root (PR) plays key roles in sustaining the aerial parts and absorbing nutrient and water in soil, and is vital for plant survival. PR growth is largely established by the division of meristem cells at the meristematic zone, and subsequent elongation of the cells at the elongation zone and differentiation of the cells at the maturation zone in the root tip.1–Citation3 It is coordinately regulated by multiple hormones such as auxin, cytokinin (CTK), gibberellins, ethylene (ET), brassinosteroid (BR) and abscisic acid (ABA), and by diverse environmental cues like water and nutrient availability, and light in plants.Citation1–Citation4 Among these, auxin is extremely important. The biosynthesis, degradation, transport and distribution of auxin significantly impact PR growth.Citation1,Citation2,Citation4 Besides hormones and environmental factors, secondary messengers such as reactive oxygen species (ROS) (H2O2 and superoxide anion), nitric oxide, inositol trisphosphate (InsP3) and calcium (Ca2+) are crucial for the regulation of PR development.Citation5–Citation8
Ca2+ as a nutrient and a signal molecule plays pivotal roles in regulating stomatal movement, responses to various biotic and abiotic stresses, and growth and development including root morphogenesis in plants.Citation6,Citation9-Citation11 It acts alone or through activating specific calcium sensors, for example, calmodulins, calcineurin B-like proteins (CBLs), calcium-dependent protein kinases (CPKs) and other Ca2+ binding proteins.Citation9,Citation12 Accumulating evidence reveals that Ca2+ is essential for PR growth.Citation13–Citation17 Previous studies have shown that Ca2+ participates in auxin-controlled maintenance of the quiescent center, and inositol trisphosphate-stimulated Ca2+ signaling affects auxin transport and Pin-FORMED (PIN) polarity during root growth of plants.Citation5,Citation6,Citation18 In this review, we discuss recent advances in the roles and regulatory mechanisms of Ca2+ in plant PR development under normal and stressful conditions.
Ca2+ regulates PR growth through affecting or mediating auxin signaling
Auxin is the central regulator of PR development. Its local accumulation and gradients in the growing root tip (with a maximum at the quiescent center) determine the maintenance and development of root apical meristem (RAM), from which root cells are produced. Auxin stimulates cell proliferation in the RAM.Citation1,Citation2 Its accumulation and gradient formation depend on the auxin efflux transporters PINs and influx transporters AUX1/Like AUX1. Auxin at a high concentration induces SCFTIR1-mediated degradation of Aux/IAA transcriptional repressors. The Aux/IAA degradation releases its binding proteins auxin response factors (ARFs), further activating downstream auxin-responsive genes to initiate diverse cellular responses.Citation19,Citation20
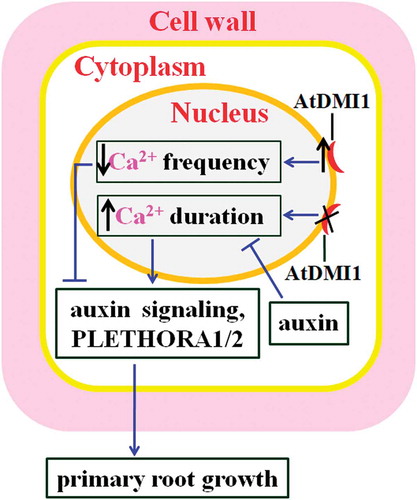
There exist complex interactions between Ca2+ and auxin during root growth. Ca2+ impacts PR development through modulating auxin accumulation, transport and signaling, as well as the expression of many auxin-related genes. Ca2+ also functions as a transducer of auxin signaling during PR elongation. It has been demonstrated that nuclear calcium signatures influence auxin signaling, altering PR development. Leitão et al found that nuclear Ca2+ signals generated in meristematic cell regulate PR growth in Arabidopsis.Citation16 Ca2+ signals (Ca2+ signatures) are modulated by nuclear membrane-localized Ca2+ channels DOES NOT MAKE INFECTIONS 1 (AtDMI1). Increases in the expression of AtDMI1 decrease the frequency of Ca2+ spikes, resulting in shorter PRs. By contrast, disruption of AtDMI1 increases the duration of Ca2+ spikes, causing longer PRs. Further study showed that AtDMI1 is required for PR growth. AtDMI1 negatively affects auxin accumulation and signaling, and the transcription of genes required for auxin biosynthesis, transport, and signaling in the RAM. These genes include Gretchen Hagen3 (GH3) gene GH3.8 (encoding the enzyme catalyzing the synthesis of IAA amide conjugates), PURIN7 (encoding 5ʹ-phosphoribosyl-4- (N-succinocarboxamide)-5-aminoimidazole synthetase), PINOID (encoding AGCVIII kinase) and PIN1. Auxin also modulates nuclear Ca2+ signals.Citation16 Thus, nuclear Ca2+ signals integrate growth-related and auxin-dependent signals to regulate PR growth in Arabidopsis ().
Figure 1. Mechanisms for nuclear Ca2+ regulating PR growth. Overexpression of AtDMI1 decreases Ca2+ frequency, inhibiting auxin signaling, decreasing the expression of PLETHORA1 and PLETHORA2, and retarding PR growth. Impairment of AtDMI1 increases Ca2+ duration, stimulating auxin signaling and the expression of PLETHORA1 and PLETHORA2, and PR growth. Thick up and down arrows, respectively, represent increases and decreases in their levels; thin arrows indicate positive regulation, and bars indicate negative regulation. The black cross means impairment of AtDMI1.
Ca2+-activated CBL-interacting protein kinase 25 (CIPK25) also positively regulates root meristem development by affecting auxin signalings.Citation17 CIPKs and CBLs are crucial signaling modules that relay calcium signals, and phosphorylate downstream targets to modulate plant adaptations to diverse environmental stresses, and growth and development including root growth.Citation9,Citation12,Citation21 It has been reported that Arabidopsis mutant cipk25 exhibits short PR phenotype and has a slower root growth rate with fewer meristem cells. Overexpression of CIPK25 causes the opposite root phenotype to cipk25. The cipk25 lines have reduced acropetal and basipetal auxin transport, decreased responsiveness to auxin-evoked root growth inhibition, and arrested auxin-responsive promoter activity. Moreover, mutation of CIPK25 leads to clear reduction of the expression of auxin-responsive genes including PIN1, PIN2, PIN3 and PIN7, and ARF5, ARF6 and ARF8, but causes the increase in the expression of short hypocotyl 2 (SHY2), one of key member of Aux/IAA family (IAA3).Citation17 Further studies revealed that disruption in SHY2 effectively rescues the short root phenotype of cipk25. Moreover, CIPK25 interacts with CBL4 and CBL5, suggesting that Ca2+ may activate CBL4 and CBL5, further motivates CIPK25 and modulates PR growth in Arabidopsis (). Additionally, chickpea CIPK25 (CaCIPK25) has shown to facilitate PR development after being overexpressed in tobacco,Citation22 reflecting the conservative function of CIPK25 in the regulation of root growth in plants.
Figure 2. Mechansims for cytosolic Ca2+ modulating PR growth. Cytosolic Ca2+ signalings regulate and mediate the signalings of auxin, CTK, BR, ABA, ET, and Cd and salt stress to affect PR development. Ca2+ regulates CBL4/5 and CIPK25, influencing auxin transport and response, and promoting PR growth. CTK inhibits CIPK25, dampening PR elongation. Ca2+ elevations regulated by CNGC2 and AtrbohD/F suppress PR growth. ABA activates CPK4/6, further promotes ET biosynthesis, inhibiting PR growth. ABA activates MPK6, leading to the reduction of ROS and Ca2+ levels and shortened roots. CMI1- and AtGLR3.6-impacted increases of Ca2+ facilitate PR growth. FER-modulated Ca2+ inhibits salt-caused damage of cell-wall integrity, and positively impacts root growth. Ca2+-activated CML38 antagonizes AtRALF1, which interferes with BR signaling, modulating PR elongation. Ca2+ attenuates Cd stress-induced disorder of auxin signaling, facilitating PR growth. Different auxinic compounds like IAA, IBA, NAA and 2,4-D elicit distinct Ca2+ signalings, favoring PR development. Arrows show positive regulation, and bars indicate negative regulation. The dashed line means uncharacterized regulation. The circle with a cross in it reveals damage of cell-wall integrity.
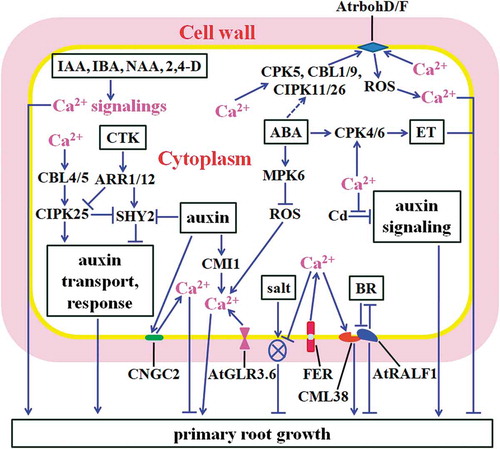
Recently, a study revealed that Ca2+-binding protein CMI1/KRP1 [Ca2+-dependent modulator of ICR1 (Interactor of constitutively active ROP1)/KIC-related protein 1] functions in the root meristem growth in Arabidopsis. It modulates auxin response and conveys auxin-triggered cytoplasmic Ca2+ signaling, positively affecting PR growth ().Citation15 Auxin has been observed to upregulate the expression of gene CMI1 through auxin receptors TIR1/AFB (Transport inhibitor1/Auxin-signaling F-Box) proteins. CMI1 defect causes the increased auxin response, altered expression of PIN2 protein, arrested increase of auxin-induced Ca2+, reduced root meristem size and shortened PRs. Moreover, CMI1 regulates auxin-elicited Ca2+ signaling in a cell type/tissue-specific manner. It acts as a monomer, and is able to bind Ca2+ at low concentrations (10−9–10−8 M), which leads to the change of its secondary structure.Citation15
In addition, CNGC2 (Cyclic nucleotide gated ion channel 2), also named DND1 (Defense, no death1), translocates Ca2+ signaling and mediates auxin responses, thereby positively impacting auxin-caused inhibition of PR growth in Arabidopsis.Citation14 CNGCs are nonselective cation channels that have been addressed to act as Ca2+ channels in plants. Loss-of-function mutant cngc2/dnd1 displays defective auxin responses and less sensitivity to auxin-repressed PR growth. The effects of the mutant can be rescued by mutation of the gene repressor of defense, no death1 (RDD1), which encodes the auxin biosynthesis gene YUCCA6. Moreover, cngc2/dnd1 exhibits clear alterations of auxin-triggered enhancement of cytosolic Ca2+.Citation14 These results support the notion that CNGC2 inhibits PR development via influencing auxin-mediated Ca2+ signaling in Arabidopsis ().
Ca2+ has also been observed to alleviate cadmium (Cd) caused PR growth inhibition by attenuating Cd-evoked disorder of auxin homeostasis in roots of Arabidopsis.Citation23 Cd treatment clearly inhibits PR elongation, and modifies the level and distribution of auxin and the expression of some genes involved in auxin biosynthetic, catabolic and transport, for example, PAT1 (PR-anthranilate transferase1), CYP79B2 (a cytochrome P450 gene), CYP79B3, NIT2 (Nitrilase2), TAA1 (Tryptophan aminotransferase1), AUX1, PIN2, PIN4, GH3.9 and GH3.17 in roots. Ca2+ application markedly alleviates or changes these effects of Cd, promoting PR growth ().Citation23 Another study indicated that Cd- or Ca2+ deficiency-induced transcription factor MYB59 negatively regulates PR growth by affecting Ca2+ homeostasis and signaling in Arabidopsis.Citation24 Yet, whether MYB59 modulates auxin homeostasis need to be further investigated.
Additionally, different cytosolic calcium signatures have been found to be induced by diverse auxin and auxinic compounds, and modulate PR growth of Arabidopsis.Citation25 Treatments with indole-3-acetic acid (IAA), indole-3-butyric acid (IBA), 1-naphthalene acetic acid (NAA), 2,4-dichlorophenoxyacetic acid (2,4-D) or mecoprop [2-(4-chloro-2-methylphenoxy) propanoic acid] lead to marked increases in cytosolic Ca2+ levels in roots and PR length (). Moreover, each of the compounds triggers a specific and unique cytosolic Ca2+ signature, implying that different Ca2+ signalings evoked by distinct auxin types may have distinguishing effects on PR growth in plants.Citation25
Ca2+ modulates PR development by impacting CTK signaling
CTK is a crucial modulator of PR growth. It suppresses RAM growth by promoting cell differentiation.Citation26 In Arabidopsis, CTK signaling is mainly mediated by histidine kinases, which activate Arabidopsis response regulators (ARRs), further stimulating the expression of CTK target genes and initiating downstream responses.Citation26 CTK regulates root growth through interacting with auxin. CTK activates ARR1, which upregulates the expression of SHY2, resulting in the inhibition of ARF activity, downregulation of PINs and repression of root cell division. Conversely, auxin facilitates SHY2 degradation, activates ARFs, and stimulates expression of PINs and cell division.Citation26,Citation27 CTK-dependent auxin degradation also contributes to root growth regulation in plants.Citation28 In Arabidopsis, CIPK25 has shown to have positive roles in PR growth.Citation17 Exogenous application of CTK decreases the expression of CIPK25, which is dependent upon ARR1 and ARR12, two important components of CTK signaling in controlling root meristem size. Moreover, loss-of-function of CIPK25 promotes the expression of SHY2, and SHY2 mutation completely rescues the short root phenotype of cipk25 (). Treatment with CTK also results in further reduction of PIN1 expression level in cipk25 relative to wild type (WT), in agreement with CTK-caused additional decrease of meristematic cell number in cipk25 compared with WT plants.Citation17 These results indicate that CIPK25 promotes root growth through integrating CTK and auxin signalings ().
Ca2+ positive impacts PR elongation through interfering with BR signaling
BR is an important hormone for PR development in plants.Citation29 Ca2+ promotes PR growth by influencing BR signaling.Citation30,Citation31 Ca2+-regulated calmodulin-like protein 38 (CML38), a secreted protein, has been addressed to physically interact with rapid alkalinization factor 1 (AtRALF1), a small secreted peptide hormone in Arabidopsis. CML38 promotes but AtRALF1 suppresses PR elongation.Citation30–Citation32 CML38 antagonizes AtRALF1-mediated root inhibition in Ca2+-dependent but not pH-dependent manner.Citation31 The transgenic plants overexpressing AtRALF1 exhibit reduced root cell size, and AtRALF1 silencing leads to the opposite effects. BR reagent brassinolide (BL) promotes PR growth at concentrations of 0.01, 0.1, and 1 nM in WT plants of Arabidopsis. However, root growth of AtRALF1 overexpressors is less sensitive to these doses of BL whereas that of AtRALF1-silenced plants is sensitive to BL. Moreover, AtRALF1 stimulates the expression of two BR-downregulated genes implicated in BR biosynthetic pathway: the cytochrome P450 monooxygenases constitutive photomorphism and dwarfism and dwarf4. BL treatment reduces the transcriptional levels of AtRALF1-inducible genes like proline rich protein 1 (AtPRP1), AtPRP3, hydroxyproline-rich glycoprotein 2 (AtHRPG2) and xyloglucan endotransglucosylase4 (TCH4).Citation30 Hence, Ca2+ affects the activity of AtRALF1 through activation of CML38, further modulating BR signaling and PR growth ().
Ca2+ serves roles in ABA-inhibited PR growth via transducing ROS signals or affecting ethylene biosynthesis
ROS has been addressed to play critical roles in the regulation of stomatal closure, adaptations to diverse biotic and abiotic stress, and plant growth and development.Citation33–Citation36 They also mediate hormone and stress signalings, and regulate RAM and PR development.Citation37–Citation39 ROS are generated through both enzymic and non-enzymic reactions in plants. Plasma membrane NADPH oxidases, also known as respiratory burst oxidase homologues (Rbohs), have been documented to be important producers of ROS.Citation40 Rbohs are involved in the regulation of numerous biological processes including stomatal movement, responses to pathogen attack and various abiotic stresses, and growth and development in plants.Citation41–Citation44 They are also key regulators of root growth.Citation13,Citation37,Citation44,Citation45 Rbohs possess two Ca2+-binding EF-hand motifs in the cytoplasmic N-terminal region that regulate their activity by Ca2+. Rbohs also promote the increase in cytoplasmic Ca2+ levels in guard cells and root cells in ABA signaling.Citation46,Citation47 Ca2+ mediates ROS signaling to positively influence ABA-inhibited PR growth.Citation13 In Arabidopsis, ABA-repressed PR elongation is impaired in the double mutants of NADPH oxidases AtrbohD and AtrbohF.Citation48 Moreover, disruptions of AtrbohD and AtrbohF clearly abolish ABA-promoted ROS generation, cytosolic Ca2+ increases, and the activation of plasma membrane Ca2+-permeable channels. Exogenous H2O2 effectively activates the Ca2+ currents in PRs of AtrbohD and AtrbohF mutant seedlings, demonstrating that Ca2+ functions downstream of ROS to regulate ABA-inhibited PR growth.Citation13 Differently, Han et al found that Arabidopsis mitogen-activated protein kinase 6 (MPK6) negatively affects ABA- or moderate H2O2-induced increases in cytosolic Ca2+, further facilitating the inhibition of cell expansion of PRs by ABA or H2O2.Citation49 That is, Ca2+ is negatively correlated with PR elongation suppressed by ABA (). The reason and underlying mechanism for the discrepancy of the role of Ca2+ in the two studies need to be further investigated.
Evidence revealed that the activities of AtrbohD and AtrbohF are modulated by Ca2+ and Ca2+ sensors.Citation50 In Arabidopsis, AtrbohD is phosphorylated and activated by CPK5 during innate immune responses, and AtrbohF is subject to regulation by CBL1, CBL9, CIPK26 and CIPK11.Citation50–Citation52 Both CIPK26 and CIPK11 interact and phosphorylate AtrbohF. Co-expression of CIPK26 with CBL1 or CBL9 and co-expression of CIPK11 with CBL1 or CBL9 clearly increase ROS generation by AtrbohF in a human embryo kidney cell line HEK293T. Moreover, direct Ca2+ binding to AtrbohF serves a role in the activation of the NADPH oxidase by phosphorylation of CBL1/CIPK26 or CBL1/CIPK11 complexes in HEK293T cells.Citation50 Yet, whether CPK5, CBL1, CBL9, CIPK26 and CIPK11 contribute to ABA-inhibited PR growth through activating AtrbohD and AtrbohF needs to be addressed in future studies.
Besides, ET signaling and level exert effects on ABA inhibition of PR growth.Citation53 ABA is able to activate CPK4 and CPK11, which phosphorylate and stabilize ACC (1–aminocyclopropane-1–carboxylate synthase) synthase 6, one of the key enzymes of ET biosynthesis, further increasing ET production in Arabidopsis.Citation54 Therefore, Ca2+ may positively modulate ABA-suppressed PR elongation by enhancing the activities of CPK4 and CPK11 and biosynthesis of ET in plants.
Ca2+ facilitates PR growth by regulating cell wall reformation
In plants, the expansion of cells including PR cells has to undergo the relaxation of cell walls without the loss of cell integrity.Citation55 Therefore, the maintenance of cell-wall integrity is essential for root growth under normal especially stress conditions. Ca2+ signal has been demonstrated to exert effects in maintaining cell-wall integrity and modulates PR elongation under salt stress.Citation56 In the presence of high concentration of salt, PR growth of Arabidopsis WT plants is inhibited; however, PRs from the mutant of gene FERONIA (FER), which encodes a receptor kinase localized in plasma-membrane, are defective. Further studies suggest that FER is responsible for the maintenance of cell-wall integrity under salinity stress. FER interacts directly with pectin, which protects cell walls against saline stress-damage through cross-linking. Importantly, FER triggers cell-specific cytosolic Ca2+ transients that are necessary to maintain cell-wall integrity under salt stress ().Citation56 Accordingly, Ca2+ signal promotes PR growth through facilitating pectin cross-linking under saline condition.
In previous studies, CML38 has shown to interplay with and counteract AtRALF1-inhibited PR growth in Arabidopsis. Moreover, AtRALF1 is able to induce the expression of genes involved in cell-wall rearrangement including AtPRP1, AtPRP3, AtHRPG2 and TCH4,Citation30,Citation31 highlighting the important role of the CML38-relayed Ca2+ signal in regulating cell wall reformation and PR development.
Other Ca2+-dependent mechanisms involved in PR growth
Experimental data suggest that Arabidopsis Ca2+-ATPase 8 (ACA8) mediates Ca2+ signaling, coordinating PR development in the absence of sucrose.Citation57 ACA8 is localized in the plasma membrane, and plays a role in the establishment of the root meristem when sucrose is unavailable. ACA8 downregulates the expression of cell cycle regulator genes, for instance, CYCB (Cyclin B), CYCD and CDKB in roots under sucrose deprivation.Citation57
Ca2+ signal may affect PR growth by modulating PLETHORA1 and PLETHORA2, two essential regulators of root development. It is because that AtDMI1, the regulator of nuclear Ca2+ signature, downregulates the expression of PLETHORA1 and PLETHORA2 during PR growth in Arabidopsis ().Citation16
Ca2+ may also function in PR development controlled by glutamate receptor-like protein AtGLR3.6 in Arabidopsis.Citation58 AtGLR3.6 positively modulates root meristem size and PR growth by altering cell division and negatively impacting the level of cyclin-dependent kinase inhibitor Kip-related protein 4 (KRP4) in an auxin-dependent manner. Mutation in AtGLR3.6 causes retarded PR growth and upregulated expression of KRP4. Furthermore, disruption of AtGLR3.6 results in significant reduction of cytosolic Ca2+ elevation in roots. Ca2+ application decreases the abundances of KRP4 and induces PR elongation, indicating that Ca2+ signal plays a role in AtGLR3.6-stimulated PR growth in Arabidopsis.Citation58 However, the relationship of Ca2+ and auxin signaling during root growth was not investigated.
In summary, Ca2+ mediates the signalings of multiple hormones particularly auxin, environmental cues and plant development to regulate PR growth. Changes in Ca2+ levels and Ca2+ signature in both the nucleus and cytoplasm have great contributions to the development of PRs. Numerous proteins include Ca2+-permeable channels, CBLs, CIPKs, CPKs, CMLs, Ca2+-binding proteins and other proteins are involved in the modulation of Ca2+ signalings and root growth ( and ). Despite these progresses, many open questions about Ca2+ affecting PR development of plants remain. For instance, how does nuclear Ca2+ coordinate cytosolic Ca2+ during root morphogenesis? What are the main sources of cytosolic Ca2+ (apoplast or diverse organelles)? What is the detailed relationship between Ca2+ concentrations, Ca2+ signal property and PR development? Moreover, how does Ca2+ simultaneously coordinate multiple different internal and external cues to regulate PR growth? What are the underlying mechanisms? These questions need to be thoroughly investigated in the coming days.
Disclosure of potential conflicts of interest
The authors declare that they have no conflicts of interest in the study.
Additional information
Funding
References
- Petricka JJ, Winter CM, Benfey PN. Control of Arabidopsis root development. Ann Rev Plant Biol. 2012;63:1–6.
- Motte H, Vanneste S, Beeckman T. Molecular and environmental regulation of root development. Ann Rev Plant Biol. 2019;70:465–488.
- Waidmann S, Sarkel E, Kleine-Vehn J. Same same, but different: growth responses of primary and lateral roots. J Exp Bot. 2020. doi:10.1093/jxb/eraa027.
- Pacifici E, Polverari L, Sabatini S. Plant hormone cross-talk: the pivot of root growth. J Exp Bot. 2015;66:1113–1121.
- Zhang J, Vanneste S, Brewer PB, Michniewicz M, Grones P, Kleine-Vehn J, Löfke C, Teichmann T, Bielach A, Cannoot B, et al. Inositol trisphosphate-induced Ca2+ signaling modulates auxin transport and PIN polarity. Dev Cell. 2011;20:855–866.
- Vanneste S, Friml J. Calcium: the missing link in auxin action. Plants. 2013;2:650–675.
- Tsukagoshi H. Control of root growth and development by reactive oxygen species. Curr Opin Plant Biol. 2016;29:57–63.
- Prakash V, Vishwakarma K, Singh VP, Rai P, Ramawat N, Tripathi KD, Sharma S. NO and ROS implications in organization of root system architecture. Plant Cell Environ. 2019. doi:10.1111/ppl.13050.
- Kudla J, Becker D, Grill E, Hedrich R, Hippler M, Kummer U, Parniske M, Romeis T, Schumacher K. Advances and current challenges in calcium signaling. New Phytol. 2018;218:414–431.
- Liu J, Niu Y, Zhang J, Zhou Y, Ma Z, Huang X. Ca2+ channels and Ca2+ signals involved in abiotic stress responses in plant cells: recent advances. Plant Cell Tissue Organ Cult. 2018;132:413–424.
- Thor K. Calcium—nutrient and messenger. Front Plant Sci. 2019;10:440.
- Medvedev SS. Principles of calcium signal generation and transduction in plant cells. Russ J Plant Physl+. 2018;65:771–783.
- Jiao YH, Sun LR, Song YL, Wang LM, Liu LP, Zhang LY, Liu B, Li N, Miao C, Hao FS. AtrbohD and AtrbohF positively regulate abscisic acid inhibited primary root growth by affecting Ca2+ signaling and auxin response of roots in Arabidopsis. J Exp Bot. 2013;64:4183–4192.
- Chakraborty S, Toyota M, Moeder W, Chin K, Fortuna A, Champigny M, Vanneste S, Gilroy S, Beeckman T, Yoshioka K. A novel role for cyclic nucleotide-gated ion channel 2 (DND1) in auxin signaling. BioRxiv. 2018. doi:10.1101/508572.
- Hazak O, Mamon E, Lavy M, Sternberg H, Behera S, Schmitz-Thom I, Bloch D, Dementiev O, Gutman I, Danziger T, et al. A novel Ca2+-binding protein that can rapidly transduce auxin responses during root growth. PLoS Biol. 2019;17(7):e3000085.
- Leitão N, Dangeville P, Carter R, Charpentier M. Nuclear calcium signatures are associated with root development. Nat Commun. 2019;10:4865.
- Meena MK, Vishwakarma NK, Tripathi V, Chattopadhyay D. CBL-interacting protein kinase 25 contributes to root meristem development. J Exp Bot. 2019;70:133–147.
- Goh CS, Lee Y, Kim S-H. Calcium could be involved in auxin-regulated maintenance of the quiescent center in the Arabidopsis root. J Plant Biol. 2012;55:143–150.
- Leyser O. Auxin signaling. Plant Physiol. 2018;176:465–479.
- Gallei M, Luschnig C, Friml J. Auxin signalling in growth: schrodinger’s cat out of the bag. Curr Opin Plant Biol. 2019;53:43–49.
- Lu TT, Zhang GF, Sun LR, Wang J, Hao FS. Genome-wide identification of CBL family and expression analysis of CBLs in response to potassium deficiency in cotton. PeerJ. 2017;5:e3653.
- Meena MK, Ghawana S, Dwivedi V, Roy A, Chattopadhyay D. Expression of chickpea CIPK25 enhances root growth and tolerance to dehydration and salt stress in transgenic tobacco. Front Plant Sci. 2015;6:683.
- Li P, Zhao C, Zhang Y, Wang X, Wang X, Wang J, Wang F, Bi Y. Calcium alleviates cadmium-induced inhibition on root growth by maintaining auxin homeostasis in Arabidopsis seedlings. Protoplasma. 2016;253:185–200.
- Fasani E, DalCorso G, Costa A, Zenoni S, Furini A. The Arabidopsis thaliana transcription factor MYB59 regulates calcium signalling during plant growth and stress response. Plant Mol Biol. 2019;99:517–534.
- Teaster ND, Sparks JA, Blancaflor EB, Hoagland RE. Interactions of auxinic compounds on a Ca2+ signaling and root growth in Arabidopsis thaliana. Am J Plant Sci. 2015;6:2989–3000.
- Wybouw B, Rybel B. Cytokinin – a developing story. Trends Plant Sci. 2019;24:177–185.
- Liu J, Moore S, Chen C, Lindsey K. Crosstalk complexities between auxin, cytokinin, and ethylene in Arabidopsis root development: from experiments to systems modeling, and back again. Mol Plant. 2017;10:1480–1496.
- Di Mambro R, De Ruvo M, Pacifici E, Salvi E, Sozzani R, Benfey PN, Busch W, Novak O, Ljung K, Di Paola L, et al. Auxin minimum triggers the developmental switch from cell division to cell differentiation in the Arabidopsis root. Proc Natl Acad Sci USA. 2017;114:7641–7649.
- Wei Z, Li J. Brassinosteroids regulate root growth, development, and symbiosis. Mol Plant. 2016;9:86–100.
- Bergonci T, Ribeiro B, Ceciliato PHO, Guerrero-Abad JC, Silva-Filho MC, Moura DS. Arabidopsis thaliana RALF1 opposes brassinosteroid effects on root cell elongation and lateral root formation. J Exp Bot. 2014;65:2219–2230.
- Campos WF, Dressano K, Ceciliato PHO, Guerrero-Abad JC, Silva AL, Fiori CS, Morato Do Canto A, Bergonci T, Claus LAN, Silva-Filho MC, et al. Arabidopsis thaliana rapid alkalinization factor 1-mediated root growth inhibition is dependent on calmodulin-like protein 38. J Biol Chem. 2018;293:2159–2171.
- Matos JL, Fiori CS, Silva-Filho MC, Moura DS. A conserved dibasic site is essential for correct processing of the peptide hormone AtRALF1 in Arabidopsis thaliana. FEBS Lett. 2008;582:3343–3347.
- Song Y, Miao Y, Song CP. Behind the scenes: the roles of reactive oxygen species in guard cells. New Phytol. 2014;201:1121–1140.
- Chan Z, Yokawa K, Kim WY, Song CP. ROS regulation during plant abiotic stress responses. Front Plant Sci. 2016;7:1536.
- Mhamdi A, Breusegem FV. Reactive oxygen species in plant development. Development. 2018;145:dev164376.
- Qi J, Song CP, Wang B, Zhou J, Kangasjärvi J, Zhu JK, Gong Z. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J Integr Plant Biol. 2018;60:67–88.
- Ma X, Zhang X, Yang L, Tang M, Wang K, Wang L, Bai L, Song CP. Hydrogen peroxide plays an important role in PERK4-mediated abscisic acid-regulated root growth in Arabidopsis. Funct Plant Biol. 2018;46:165–174.
- Sun LR, Zhao ZJ, Hao FS. NADPH oxidases, essential players of hormone signalings in plant development and response to stresses. Plant Signal Behav. 2019;14:e1657343.
- Lee Y. Redox control on stem cell fate and maintenance in the root. J Plant Biol. 2019;62:320–328.
- Zhang G, Yue C, Lu T, Sun L, Hao F. Genome-wide identification and expression analysis of NADPH oxidase genes in response to ABA and abiotic stresses, and in fibre formation in Gossypium. PeerJ. 2020;8:e8404.
- Ma LY, Zhang H, Sun LR, Jiao YH, Zhang GZ, Miao C, Hao FS. NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na+/K+ homeostasis in Arabidopsis under salt stress. J Exp Bot. 2012;63:305–317.
- Liu B, Sun LR, Ma LY, Hao FS. Both AtrbohD and AtrbohF are essential for mediating responses to oxygen deficiency in Arabidopsis. Plant Cell Rep. 2017;36:947–957.
- Sun LR, Ma LY, He SB, Hao FS. AtrbohD functions downstream of ROP2 and positively regulates waterlogging response. Plant Signal Behav. 2018;13:e1513300.
- Chen Q, Yang G. Signal function studies of ROS, especially RBOH dependent ROS, in plant growth, development and environmental stress. J Plant Growth Regul. 2019. doi:10.1007/s00344-019-09971-4.
- Li N, Sun LR, Zhang LY, Song YL, Hu PP, Li C, Hao FS. AtrbohD and AtrbohF negatively regulate lateral root development by changing the localized accumulation of superoxide in primary roots of Arabidopsis. Planta. 2015;241:591–602.
- Demidchik V, Shabala S. Mechanisms of cytosolic calcium elevation in plants: the role of ion channels, calcium extrusion systems and NADPH oxidase-mediated ‘ROS-Ca2+ Hub’. Funct Plant Biol. 2017;45:9–27.
- Chen K, Li G, Bressan RA, Song C, Zhu J, Zhao Y. Abscisic acid dynamics, signaling and functions in plants. J Integr Plant Biol. 2020;62:25–54.
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. Embo J. 2003;22:2623–2633.
- Han S, Fang L, Ren X, Wang W, Jiang J. MPK6 controls H2O2-induced root elongation by mediating Ca2+ influx across the plasma membrane of root cells in Arabidopsis seedlings. New Phytol. 2015;205:695–706.
- Han JP, Koster P, Drerup MM, Scholz M, Li S, Edel KH, Hashimoto K, Kuchitsu K, Hippler M, Kudla J. Fine-tuning of RBOHF activity is achieved by differential phosphorylation and Ca2+ binding. New Phytol. 2019;221:1935–1949.
- Dubiella U, Seybold H, Durian G, Komander E, Lassig R, Witte CP, Schulze WX, Romeis T. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc Natl Acad Sci USA. 2013;110:8744–8749.
- Drerup MM, Schl€ucking K, Hashimoto K, Manishankar P, Steinhorst L, Kuchitsu K, Kudla J. The Calcineurin B-like calcium sensors CBL1 and CBL9 together with their interacting protein kinase CIPK26 regulate the Arabidopsis NADPH oxidase RBOHF. Mol Plant. 2013;6:559–569.
- Qin H, He L, Huang R. The coordination of ethylene and other hormones in primary root development. Front Plant Sci. 2019;10:874.
- Luo X, Chen Z, Gao J, Gong Z. Abscisic acid inhibits root growth in Arabidopsis through ethylene biosynthesis. Plant J. 2014;79:44–55.
- Vaahtera L, Schulz J, Hamann T. Cell wall integrity maintenance during plant development and interaction with the environment. Nat Plants. 2019;5:924–932.
- Feng W, Kita D, Peaucelle A, Cartwright HN, Doan V, Duan Q, Liu M-C, Maman J, Steinhorst L, Schmitz-Thom I, et al. The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr Biol. 2018;28:666–675.e5.
- Zhang J, Zhang X, Wang R, Li W. The plasma membrane-localised Ca2+-ATPase ACA8 plays a role in sucrose signalling involved in early seedling development in Arabidopsis. Plant Cell Rep. 2014. doi:10.1007/s00299-014-1590-y.
- Singh SK, Chien CT, Chang IF. The Arabidopsis glutamate receptor-like gene GLR3.6 controls root development by repressing the Kip-related protein gene KRP4. J Exp Bot. 2016;67:1853–1869.
