ABSTRACT
A rice COBRA-like gene, BRITTLE CULM1 (BC1) has been shown to be involved in assembling cell wall components and cellulose crystallinity, which determines mechanical strength in above ground organs. However, the detailed roles of BC1 in rice development are poorly understood. In this study, we found that, unlike the known brittle culm mutants, the internode length of the bc1 mutant was ~1.27 times longer than that of wild type in rice. In order to analyze the effects of bc1 mutation on internode development, we compared the deposition of cell wall components among each developmental stage of the elongating second internodes from wild type, Kinmaze, and the bc1 mutant. In wild type, histochemical observations of lignin revealed that lignin deposition was gradually increased after the cell elongation stage of the internodes. Cellulose and p-coumaric acid (pCA) content also gradually increased along with the progress of the developmental stage. The ferulic acid (FA) content rapidly increased in the cell elongation stage and decreased at the late secondary cell wall formation stage. In the bc1 mutant, the contents of cell wall components were lower than those of wild type from the cell elongation stage, in which the BC1 started to express at this stage in wild type. In the bc1 mutant, the deposition patterns of cell wall components, especially phenolic components including lignin, pCA, and FA, were delayed compared with those of wild type. These results suggest that the BC1 gene plays a role in synthesizing appropriate cell walls at each stage in the developing internode.
KEYWORDS:
Introduction
The plant cell wall is a rigid and dynamic network system that is involved in the regulation of cell division and expansion, defense response, and determination of shape and mechanical properties of organs.Citation1–Citation3 In rice internodes that compose rice stems (also known as culm), the cell wall provides important traits, including suitable length and mechanical properties, to support high-yield panicles. In rice plants, the internodes rapidly elongate during the heading stage, which involves various biological processes, such as cell elongation, cell elongation arrest, and secondary cell wall synthesis. Generally, the basal part of the elongating internode in grass plants consists of young cells, including dividing and elongating cells, and these cells mature toward the apical parts by forming secondary cell walls.Citation4,Citation5
Grass cell walls, including rice, have Type II cell walls that differ from those of dicotyledonous plants. Type II cell walls characteristically contain low molecular hydroxycinnamates, especially ferulic acid (FA) and p-coumaric acid (pCA), which are ester-linked to the primary and secondary cell walls.Citation6–Citation8 The primary cell walls are deposited during cell division and expansion and have a strong and plastic fiber composite that responds to requirements during cell growth.Citation9 In rice, the primary cell wall is mainly composed of cellulose, hemicelluloses, and low molecular hydroxycinnamates. In contrast, the secondary cell walls are synthesized after cessation of cell expansion, which largely determines the mechanical strength of plants and composes the bulk of global biomass.Citation10,Citation11 The secondary cell wall mainly contains cellulose, hemicelluloses, and lignin, and coordinated deposition and proper assembly of these polymers are needed for determining plant shape and mechanical strength.
In rice, several brittle culm (bc) mutants with decreased mechanical strength of the internodes have been shown to exhibit different types of gene defects responsible for cell wall biosynthesis. Most bc mutants showed decreased cellulose content of the secondary cell wall, indicating that cellulose is one of the important components for maintaining the mechanical strength of rice internodes.Citation12–Citation16 In fact, bc6, Citation17 bc7, Citation18 and bc11Citation19 have defects in CesA genes responsible for cellulose biosynthesis in the rice secondary cell wall. Among several rice bc mutants, the bc1 mutant exhibited a characteristic cell wall structure. The bc1 had decreased cellulose content, increased lignin content, Citation13 perturbed cell wall deposition in sclerenchyma with local aggregation of the secondary cell wall materials,Citation14,Citation20 and decreased crystallinity of cellulose microfibril in the internodes.Citation21
BC1 encodes COBRA-like glycosyl phosphatidylinositol (GPI) anchored protein.Citation13 The N-terminal amino acid sequence of BC1 shows weak similarity to the carbohydrate-binding module (CBM), and this CBM-like sequence has been shown to be necessary for proper BC1 function.Citation20 A recent report has shown that the CBM-like sequence specifically interacts with crystalline cellulose and that BC1 protein localizes the cell wall region.Citation21 Therefore, it has been suggested that BC1 plays an important role in assembling cell wall materials and crystalline cellulose, though the detailed effects of bc1 mutation on plant development are poorly understood.
In this study, we found that the mutation of the bc1 gene induced longer internodes than those of wild type. Therefore, we analyzed the cell wall deposition pattern in each developmental stage of the elongating internodes from wild type, Kinmaze, and the bc1 mutant. We divided the elongating second internode into four developmental stages based on histochemical analysis: before the cell elongation stage, the cell elongation stage, and early and late secondary cell wall development stages. Our results demonstrated that the deposition of cell wall components, especially phenolic components such as lignin, FA, and pCA esters, were delayed in the bc1 mutant compared to wild type. The distinct deposition pattern in bc1 was not observed at the stage before cell elongation, and it appeared in the cell elongation stage in which the BC1 gene was shown to have been expressed. These results suggest that the original BC1 gene has a function in synthesizing the appropriate cell wall at each developmental stage during internode elongation, which would affect internode development.
Materials and methods
Plant materials and growth conditions
The rice (Oryza sativa L.) brittle culm1 mutant was isolated from japonica cultivar Kinmaze. The rice plants used in this study were cultivated in a greenhouse under natural conditions from April to September.
Histochemical and anatomical observation of lignin
The rice internodes were numbered from top to bottom as previously described.Citation22 Samples of second internodes during elongation were harvested just before ear heading. These samples were divided into 10 segments at 0.5 cm intervals from the base of the internodes. For light microscopy, segments from the second internodes were fixed in formalin:acetic acid:70% ethanol (1:1:18) overnight at room temperature. To stain the lignin in the internodes, phloroglucinol reactions were used. For the phloroglucinol reaction, 60-µm sections were prepared with a sliding microtome (LS113; Yamato Koki, Saitama, Japan), incubated for 2 min in phloroglucinol solution (2% in ethanol), treated with a few drops of 12 M hydrochloric acid, and then observed with a light microscope (DMLB; Leica, Wetzler, Germany).
For anatomical observation, each fixed segment was dehydrated through a graded ethanol series (50%, 60%, 70%, 80%, 95%, and finally 100% twice, for at least 1 h in each case) and then embedded in Paraplast Plus paraffin wax (McCormick Scientific, St Louis, MO, USA). Ten-µm sections were cut with a carbon knife on a microtome and stained with 0.25% toluidine blue O (Sigma-Aldrich Japan, Tokyo, Japan) and observed with the light microscope.
Expression analysis of BC1 gene
Total RNA was extracted with an RNeasy plant mini kit (Qiagen, Hilden, Germany) from each stage of the elongating internode of wild type, Kinmaze, and bc1 mutant. The first-strand cDNA was synthesized using ReverTra Ace® RT Master Mix with gDNA Remover (Toyobo, Osaka, Japan). Reverse transcription polymerase chain reaction (RT-PCR) was performed using KOD One® PCR Master Mix under the following conditions: 94°C for 2 min, 32 cycles at 98°C for 10 s, 58°C for 5 s, 68°C for 10 s. The following primer pairs were used for amplifying BC1 and Actin as a control: BC1 F: 5ʹ-ATCGCCATCACAAACTTCAA-3ʹ and R: 5ʹ-TTTCGCATCAGCACCTCC-3ʹ and Actin F: 5ʹ-GATCACTGCCTTGGCTCCTA-3ʹ and R: 5ʹ-GTACTCAGCCTTGGCAATCC-3ʹ.
Analysis of major cell wall components
Samples of elongating second internodes for cell wall analysis were divided into four stages: cell division stage (S1: 0–0.5 cm from the base), cell expansion stage (S2: 0.5–1.5 cm from the base), early secondary cell wall formation stage (S3: 1.5–2.5 cm from the base), and late secondary cell wall formation stage (S4: 2.5–5 cm from the base). Each sample was ground into a fine powder and extracted using the modified procedure described in a previous report.Citation23 The samples were extracted with 80% ethanol at 100ºC for 1 h three times, acetone at room temperature for 10 min, and methanol at room temperature for 10 min, and then the residue was dried at 60°C for 1 to 2 d. The resultant powder was incubated in 10 mM Tris-malate buffer (pH 6.9) at 100°C for 10 min to gelatinize the starch granules, and the starch was then digested twice in α-amylase solution (2 U mg−Citation1 of carbohydrate in 10 mM Tris-malate buffer) from porcine pancreas (Merck) at 40°C for 1 h. Next, four volumes of cold absolute ethanol were added and the temperature was maintained at −20°C for 1 h. The α-amylase treated alcohol-insoluble residue (AIR) was washed three times with absolute ethanol and then dried at 60°C for 1 to 2 d. For cellulose measurement, the dried AIR (approximately 30 mg) was dissolved in acetic and nitric acidsCitation24 and completely dried in a vacuum oven. The weight of the purified crystalline celluloses was measured. Measurement of total phenolic content including lignin and cell wall-linked phenolic acids in the cell wall was performed by the acetyl bromide methodCitation25 using approximately 10-mg AIR samples.
Analysis of alkali-labile phenolics in cell wall
The levels of hydroxycinnamic acids linked to the cell walls via alkali-labile linkage, such as ester bonds, in the cell wall were measured following alkaline hydrolysis of the α-amylase-treated AIR. Approximately 20 mg of dried AIR was treated with 5 ml of 1 M sodium hydroxide at room temperature for 24 h, and subsequent experiments proceeded according to a previously described method.Citation26 Hydroxycinnamates were analyzed by gas chromatography on a capillary column DB-5 (Agilent Technologies, CA, USA).
Results and discussion
Mutation of bc1 gene affects internode length
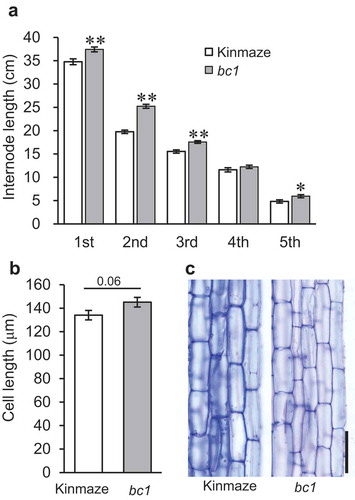
Previous reports have shown that the rice bc1 mutation affects plant mechanical strength and secondary cell wall formation.Citation13,Citation14 In this study, we noticed that the plant height of the bc1 mutant appears to be slightly taller than that of wild type, Kinmaze.Citation14 We therefore compared internode length between the wild type and the bc1 mutant. The bc1 mutant had 1.07 to 1.27 times longer internodes than wild type, especially at the upper internodes (). Furthermore, we measured elongated cell length in the secondary internode from wild type and the bc1 mutant (). The cell length of parenchyma tissues in wild type and the bc1 mutant approached significance (P = .061) (), suggesting the bc1 had 1.08 times longer parenchyma cells than those of wild type. Previous anatomical expression analyses showed that the expression of the BC1 gene has appeared not only in young mechanical tissues but also in young parenchyma tissues.Citation13,Citation14 Therefore, the mutation of bc1 could induce a slight delay of cell elongation arrest, which could result in longer internodes compared to wild type. These results suggest that the original BC1 gene has a function not only in plant mechanical strengthening but also in the process of rice internode development. In order to clarify the effects of bc1 mutation on internode development, we tried to separate the elongating internode by developmental stages in a subsequent experiment.
Figure 1. Internode and cell length in wild type, Kinmaze, and bc1 mutant. (a) Length of first to fifth internodes in wild type (white) and bc1 mutant (gray). Internodes are numbered from top to bottom. The main culm in each plant was used for measurement. Values are averages of 20 biological replicates, and bars represent standard errors. Statistical analysis was performed using Student’s t-test (**p < .01; *p < .05). (b) Length of cells at 3.5 cm from lower node in elongating second internodes. Values are averages of 50 cells (10 cells per plant), and bars represent standard errors. Statistical analysis was performed using Student’s t-test, and P value is shown at top of graph. (c) Longitudinal sections of elongating second internode at 3.5 cm from lower node in wild type, Kinmaze (left) and bc1 mutant (right). Bar = 100 μm.
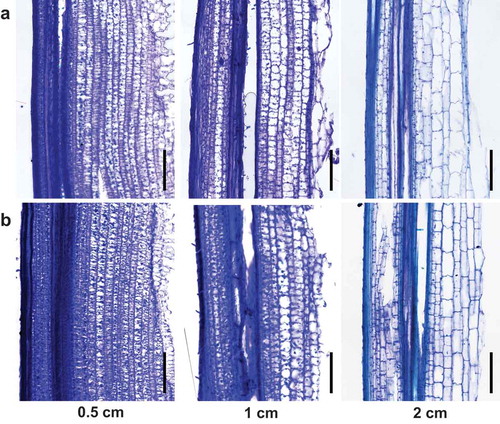
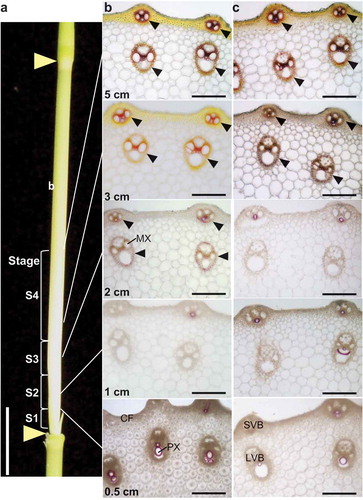
Histochemical observations of elongating rice internode
In the rice plant, the internodes rapidly elongate and develop during the heading stage. When the ear emerges from the frag leaf sheath, the second internode from the earCitation22 is just elongating. In order to characterize the cell developmental stage of the elongating internode in wild type, Kinmaze, we observed internode segments taken from each position shown in . The transverse sections of the Kinmaze internode were stained with phloroglucinol and lignin deposition was observed. In the sections at 0.5 and 1 cm from the lower node of the second internodes, red-colored phloroglucinol staining of the lignin was observed only in the protoxylem of the large and small vascular bundle (). The section at 2 cm exhibited lignin deposition in the metaxylem and the mestome sheath of the large and small vascular bundle. The phloroglucinol staining was gradually intensified in the section at 3 cm and 5 cm as the developmental stage becomes more mature. The longitudinal section stained with toluidine blue O showed uniform and flat parenchyma cells in the section at 0.5 cm from the lower node (), indicating that these cells are present before cell elongation. In the sections at 1 cm, the parenchyma cells have longitudinally longer cells than those at 0.5 cm, indicating that the parenchyma cells have started to elongate (). The section at 2 cm had vertically long parenchyma cells () with lignified vascular cells (). These observations showed that the elongating second internodes of rice contain cells in various developmental stages, which is consistent with the findings of previous reports.Citation4,Citation5 Based on the results of these histochemical observations in wild type, the elongating second internodes could divide into the following four stages: the 0–0.5 cm region including pre-elongation cells (designated as S1), the 0.5–1.5 cm region including elongating cells (S2), the 1.5–2.5 cm region including cells in early secondary cell wall formation (S3), and the 2.5–5 cm region including late secondary cell wall formation (S4) (). In the bc1 mutant, longitudinal sections from 0.5 cm to 2 cm from the lower node showed almost the same features as those of wild type (). On the other hand, the phloroglucinol staining of lignin in the vascular bundle, with the exception of protoxylems, appeared at 3 cm from the lower node (). These results suggest that the mutation of the bc1 gene induces a delay in lignin deposition in elongating internodes.
Figure 2. Histochemical observation of elongating second internode of rice. (a) Positions of sections for histochemical observation and segments of S1 to S4 for subsequent analyses are shown. Triangles indicate nodes. Bar = 2 cm. (b and c) Transverse sections stained with phloroglucinol reaction of cell wall lignin in wild type, Kinmaze (b), and bc1 mutant (c) indicating delayed lignin deposition in bc1 mutant. Arrowheads indicate phloroglucinol stained mestome sheath. Bars = 50 μm. CF, cortical fiber; LVB, large vascular bundle; MX, metaxylem; PX, protoxylem; SVB, small vascular bundle.
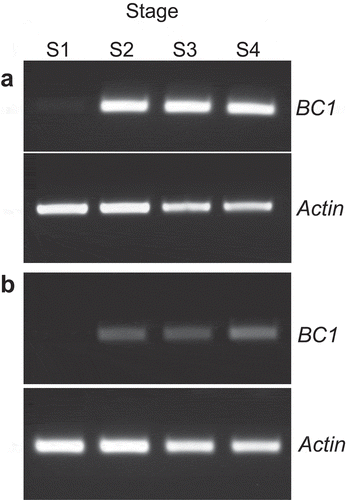
Expression of the BC1 gene in the elongating second internode
The BC1 gene expression was tested by RT-PCR with cDNA derived from each developmental stage in the elongating second internode of wild type, Kinmaze, and bc1 mutant. The Actin gene was used as a control. The BC1 gene was not amplified in the S1 region in both wild type () and bc1 (), which contains cells before cell elongation. The BC1 expression was detected from the S2 to S4 regions, in which cells were elongating and/or synthesizing secondary cell walls. Consistent with previous reports, Citation13,Citation20 a dramatic reduction in BC1 expression was induced by the bc1 mutation (). Previous histochemical analyses showed that BC1 was mainly expressed in the young mechanical tissues such as the vascular bundle and sclerenchyma cells in which thick cell walls form in the future, and the expressions disappeared in the matured tissues.Citation13,Citation14 In this study, it is confirmed that the BC1 gene is not expressed before the cell elongation stage and is subsequently highly expressed during cell elongation and secondary cell wall formation. These results indicated that the expression of the BC1 gene is strictly regulated in the developmental stage in rice internodes.
Cell wall components in each stage of the elongating internode
Previous studies showed that the bc1 mutant has an altered cell wall of mature internodes with a reduced amount of cellulose (~70% of that in the wild type) and an increased amount of lignin (~130% of that in the wild type).Citation13,Citation14 In this study, we measured these contents in different developmental stages of elongating internodes in order to clearly identify the effects of bc1 mutation on cell wall formation. Because the cellulose is the main component of both primary and secondary cell walls, the contents were expected to increase along with the cell wall formation. The cellulose content in the α-amylase-treated AIR in wild type, Kinmaze, gradually increased from the young S1 (167.6 μg/mg AIR) to older S4 (517.1 μg/mg AIR) regions (), which suggests that cell wall formation actively progressed from young to mature regions of elongating internodes. The cellulose content in the bc1 mutant also showed a gradual increase along with the developmental stage (). However, the contents were reduced to ~73% of that in the wild type from the S2 to S4 regions in which the BC1 gene is expressed in wild type rice (). Therefore, bc1 mutation has been shown to induce the reduction of cellulose contents from the cell elongation stage.
Figure 5. Contents of cell wall components at S1 to S4 stages of elongating internodes shown in Figure 2. Cellulose content per AIR (a), acetyl-bromide lignin per AIR (b), alkaline-soluble FA (c) and pCA (d) per AIR in wild type, Kinmaze (white bar), and bc1 mutant (gray bar) cell walls are shown. Values are averages of three biological replicates, and bars represent standard errors. Statistical analysis was performed using Tukey’s test. Different letters indicate significant difference at p < .05.
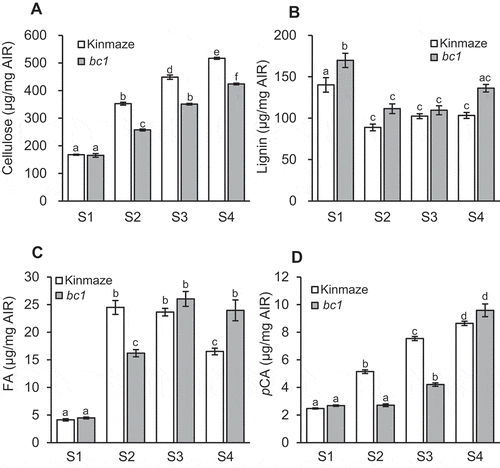
Lignin is the major component of secondary cell walls. In wild type plants, the content of acetyl-bromide lignin was the highest in S1 (140.2 μg/mg AIR) and decreased at S2 (88.8 μg/mg AIR). A significant difference was not observed between S2 and S4 (). In the S1 segment, protoxylem exhibited a matured secondary cell wall, but the cell walls of other tissues were still under construction (). It is therefore suggested that the protoxylem accounted for a large percentage in the total cell wall of S1. Similar high lignin content in the youngest internode was observed in developing maize and rice internodes.Citation5,Citation27 Although significant differences in lignin content were not observed between S2 to S4 in wild type, histochemical observation showed gradually intensified staining of lignin by phloroglucinol from the S3 to S4 regions (). In these regions, the secondary cell wall, which consists of not only lignin but also cellulose and hemicelluloses, is actively synthesized after cell elongation arrest. Therefore, the amount of lignin in the internode segment was increased along with secondary cell wall formation as shown in , even though the content of lignin per AIR was nearly constant. The bc1 mutant also showed the highest deposition of phenolic components in S1 compared with those of subsequent stages. In S1 and S4, the total phenolic contents were significantly higher than those of wild type (). The protoxylem in S1 is mature tissue after the BC1 expression has disappeared. Therefore, the higher total phenolic content in the bc1 mutant is consistent with the previous reports that showed a higher lignin content in mature internodes.Citation13,Citation14
In order to reveal the deposition pattern of other cell wall materials, we measured pCA and FA, which are ester-linked to cell wall components.Citation8,Citation28-Citation33 In wild type, the FA content rapidly increased from S1 to S2 and decreased at S4 (). In contrast, the content of pCA gradually increased from S1 to S4 (). The FA esters were rapidly synthesized and deposited during the cell elongation prior to secondary cell wall formation, and the pCA esters were actively deposited during cell elongation and secondary cell wall formation. Similar distinct deposition patterns of FA and pCA esters during internode development have been reported in maizeCitation27 and rice.Citation5 Interestingly, in the bc1 mutant, the FA content increased from the S1 to S3 regions, which was one stage behind the deposition pattern shown for wild type (). The pCA content in the bc1 mutant started to increase in the S3 region (), which also showed a delayed deposition pattern compared to that of wild type. These results suggest that the proper cell wall formation after S2 at the cell elongation stage is delayed in the bc1 mutant, which is similar to the lignin deposition pattern shown by histochemical analyses ( b, c).
To date, several types of bc mutants that exhibit decreased mechanical strength of internode cell walls have been reported. These rice mutants exhibit defects in various kinds of genes: CesA in bc6, Citation17 bc7, Citation18 and bc11 ;Citation19 dynamin-like gene in bc3 ;Citation12,Citation15 DUF266-containing and golgi-located type II membrane protein in bc10 ;Citation16 kinesin 4 in bc12 ;Citation34 and membrane-associated chitinase-like protein in bc15 .Citation35 Additionally, most of these mutants also showed decreased plant height. In this study, we showed for the first time that the bc1 mutant had longer internodes than that of wild type, which suggests that decreased mechanical strength of the cell wall should not be a direct cause of promoting internode elongation in the bc1 mutant.
BC1 has been shown to encode COBRA-like GPI-anchored protein, which directly interacts with crystalline cellulose in the cell wall and affects cellulose microfibril crystallinity.Citation13,Citation14,Citation21 In fact, the bc1 mutant showed decreased cellulose crystallinity in internodes, yet the relationship between cellulose crystallinity and internode elongation is not well understood. To date, cobra, Citation36 korrigan,Citation37 and chitinase-like1/pom-pom1Citation38 mutants in Arabidopsis have exhibited decreased cellulose crystallinity and a dwarf phenotype. In rice, the Osfc16 mutant that has amino acid substitution at w481 C and P482 S in the CesA9 gene has also exhibited decreased cellulose crystallinity and slightly shorter plant height than that of wild type.Citation39 In contrast, a decreased proportion of crystalline cellulose was observed during rapid anisotropic growth at high temperature in Arabidopsis stems.Citation40 The molecular mechanisms that regulate cellulose crystallinity and its effect on cell wall formation and plant development are still largely unknown. A more detailed understanding of the BC1 function in cellulose crystallinity, internode elongation, and cell wall formation will provide information to clarify the relationship between the regulation of cellulose crystallinity and plant development. High cellulose crystallinity makes microfibrils highly inextensible, inducing a negative effect on plant lodging resistance and enzymatic digestibility in plants.Citation41,Citation42 Grass plants are widely used not only as main grain crops but also for livestock feed and plant biomass. Therefore, the functional elucidation of BC1 in rice is expected to contribute to the improvement of lodging resistance and digestibility in grass plants for agricultural production and industrial application.
Abbreviations
| AIR | = | alcohol-insoluble residue |
| BC | = | brittle culm |
| CBM | = | carbohydrate-binding module |
| FA | = | ferulic acid |
| GPI | = | glycosyl phosphatidylinositol |
| pCA | = | p-coumaric acid |
| RT-PCR | = | reverse transcription polymerase chain reaction |
Disclosure of potential conflicts of interest
No potential conflicts of interest was disclosed.
Acknowledgments
We thank Dr. Katayama, emeritus professor of Tokyo University of Agriculture and Technology, for his helpful discussions and Kazuki Imai and Kazuaki Minegishi for their technical assistance.
References
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993;3(1):1–8. doi:10.1111/j.1365-313X.1993.tb00007.x.
- Scheible WR, Pauly M. Glycosyltransferases and cell wall biosynthesis: novel players and insights. Curr Opin Plant Biol. 2004;7(3):285–295. doi:10.1016/j.pbi.2004.03.006.
- Vorwerk S, Somerville S, Somerville C. The role of plant cell wall polysaccharide composition in disease resistance. Trends Plant Sci. 2004;9(4):203–209. doi:10.1016/j.tplants.2004.02.005.
- Kende H, van der Knaap E, Cho H-T. Deepwater rice: a model plant to study stem elongation. Plant Physiol. 1998;118(4):1105. doi:10.1104/pp.118.4.1105.
- Lin F, Williams BJ, Thangella PAV, Ladak A, Schepmoes AA, Olivos HJ, Zhao K, Callister SJ, Bartley LE. Proteomics coupled with metabolite and cell wall profiling reveal metabolic processes of a developing rice stem internode. Front Plant Sci. 2017;8:1134. doi:10.3389/fpls.2017.01134.
- Iiyama K, Lam TBT, Stone BA. Covalent cross-links in the cell wall. Plant Physiol. 1994;104(2):315–320. doi:10.1104/pp.104.2.315.
- Mueller-Harvey I, Hartley RD, Harris PJ, Curzon EH. Linkage of p-coumaroyl and feruloyl groups to cell-wall polysaccharides of barley straw. Carbohydr Res. 1986;148(1):71–85. doi:10.1016/0008-6215(86)80038-6.
- Vogel J. Unique aspects of the grass cell wall. Curr Opin Plant Biol. 2008;11(3):301–307. doi:10.1016/j.pbi.2008.03.002.
- Darley CP, Forrester AM, McQueen-Mason SJ. The molecular basis of plant cell wall extension. Plant Mol Biol. 2001;47(1):179–195. doi:10.1023/A:1010687600670.
- Boudet AM, Kajita S, Grima-Pettenati J, Goffner D. Lignins and lignocellulosics: a better control of synthesis for new and improved uses. Trends Plant Sci. 2003;8(12):576–581. doi:10.1016/j.tplants.2003.10.001.
- Jones L, Ennos AR, Turner SR. Cloning and characterization of irregular xylem4 (irx4): a severely lignin-deficient mutant of Arabidopsis. Plant J. 2001;26(2):205–216. doi:10.1046/j.1365-313x.2001.01021.x.
- Hirano K, Kotake T, Kamihara K, Tsuna K, Aohara T, Kaneko Y, Takatsuji H, Tsumuraya Y, Kawasaki S. Rice BRITTLE CULM 3 (BC3) encodes a classical dynamin OsDRP2B essential for proper secondary cell wall synthesis. Planta. 2010;232(1):95–108. doi:10.1007/s00425-010-1145-6.
- Li Y, Qian Q, Zhou Y, Yan M, Sun L, Zhang M, Fu Z, Wang Y, Han B, Pang X, et al. BRITTLE CULM1, which encodes a COBRA-like protein, affects the mechanical properties of rice plants. Plant Cell. 2003;15(9):2020. doi:10.1105/tpc.011775.
- Sato K, Suzuki R, Nishikubo N, Takenouchi S, Ito S, Nakano Y, Nakaba S, Sano Y, Funada R, Kajita S, et al. Isolation of a novel cell wall architecture mutant of rice with defective Arabidopsis COBL4 ortholog BC1 required for regulated deposition of secondary cell wall components. Planta. 2010b;232(1):257–270. doi:10.1007/s00425-010-1171-4.
- Xiong G, Li R, Qian Q, Song X, Liu X, Yu Y, Zeng D, Wan J, Li J, Zhou Y. The rice dynamin-related protein DRP2B mediates membrane trafficking, and thereby plays a critical role in secondary cell wall cellulose biosynthesis. Plant J. 2010;64(1):56–70. doi:10.1111/j.1365-313X.2010.04308.x.
- Zhou Y, Li S, Qian Q, Zeng D, Zhang M, Guo L, Liu X, Zhang B, Deng L, Liu X, et al. BC10, a DUF266-containing and golgi-located type II membrane protein, is required for cell-wall biosynthesis in rice (Oryza sativa L.). Plant J. 2009;57(3):446–462. doi:10.1111/j.1365-313X.2008.03703.x.
- Kotake T, Aohara T, Hirano K, Sato A, Kaneko Y, Tsumuraya Y, Takatsuji H, Kawasaki S. Rice Brittle culm 6 encodes a dominant-negative form of CesA protein that perturbs cellulose synthesis in secondary cell walls. J Exp Bot. 2011;62(6):2053–2062. doi:10.1093/jxb/erq395.
- Yan C, Yan S, Zeng X, Zhang Z, Gu M. Fine mapping and isolation of Bc7(t), allelic to OsCesA4. J Genet Genomics. 2007;34(11):1019–1027. doi:10.1016/s1673-8527(07)60115-5.
- Zhang B, Deng L, Qian Q, Xiong G, Zeng D, Li R, Guo L, Li J, Zhou Y. A missense mutation in the transmembrane domain of CESA4 affects protein abundance in the plasma membrane and results in abnormal cell wall biosynthesis in rice. Plant Mol Biol. 2009;71(4–5):509–524. doi:10.1007/s11103-009-9536-4.
- Sato K, Ito S, Fujii T, Suzuki R, Takenouchi S, Nakaba S, Funada R, Sano Y, Kajita S, Kitano H, et al. The carbohydrate-binding module (CBM)-like sequence is crucial for rice CWA1/BC1 function in proper assembly of secondary cell wall materials. Plant Signal Behav. 2010a;5(11):1433–1436. doi:10.4161/psb.5.11.13342.
- Liu L, Shang-Guan K, Zhang B, Liu X, Yan M, Zhang L, Shi Y, Zhang M, Qian Q, Li J, et al. Brittle Culm1, a COBRA-like protein, functions in cellulose assembly through binding cellulose microfibrils. PLoS Genet. 2013;9(8):e1003704. doi:10.1371/journal.pgen.1003704.
- Kurotani KI, Hattori T, Takeda S. Overexpression of a CYP94 family gene CYP94C2b increases internode length and plant height in rice. Plant Signal Behav. 2015;10(7):e1046667. doi:10.1080/15592324.2015.1046667.
- Pettolino FA, Walsh C, Fincher GB, Bacic A. Determining the polysaccharide composition of plant cell walls. Nat Protoc. 2012;7(9):1590–1607. doi:10.1038/nprot.2012.081.
- Updegraff DM. Semimicro determination of cellulose in biological materials. Anal Biochem. 1969;32(3):420–424. doi:10.1016/s0003-2697(69)80009-6.
- Iiyama K, Wallis AFA. Determination of lignin in herbaceous plants by an improved acetyl bromide procedure. J Sci Food Agric. 1990;51(2):145–161. doi:10.1002/jsfa.2740510202.
- Nishikubo N, Araki T, Kajita S, Kuroda K, Kitano H, Katayama Y. Specific accumulation of polysaccharide-linked hydroxycinnamoyl esters in the cell walls of irregularly shaped and collapsed internode parenchyma cells of the dwarf rice mutant Fukei 71. Plant Cell Physiol. 2000;41(6):776–784. doi:10.1093/pcp/41.6.776.
- Jung HG, Casler MD. Maize stem tissues: cell wall concentration and composition during development. Crop Sci. 2006;46(4):1793. doi:10.2135/cropsci2005.02-0085.
- Faulds CB, Mandalari G, LoCurto R, Bisignano G, Waldron KW. Arabinoxylan and mono- and dimeric ferulic acid release from brewer’s grain and wheat bran by feruloyl esterases and glycosyl hydrolases from Humicola insolens. Appl Microbiol Biotechnol. 2004;64(5):644–650. doi:10.1007/s00253-003-1520-3.
- Hatfield RD, Ralph J, Grabber JH. Cell wall cross-linking by ferulates and diferulates in grasses. J Sci Food Agric. 1999;79(3):403–407. doi:10.1002/(sici)1097-0010(19990301)79:3<403::aid-jsfa263>3.0.co;2-0.
- Ishii T, Hiroi T, Thomas JR. Feruloylated xyloglucan and p-coumaroyl arabinoxylan oligosaccharides from bamboo shoot cell-walls. Phytochemistry. 1990;29(6):1999–2003. doi:10.1016/0031-9422(90)85055-K.
- Lu F, Ralph J. Detection and determination of p-coumaroylated units in lignins. J Agric Food Chem. 1999;47(5):1988–1992. doi:10.1021/jf981140j.
- Ralph J. Hydroxycinnamates in lignification. Phytochemistry Reviews. 2010;9(1):65–83. doi:10.1007/s11101-009-9141-9.
- Saulnier L, Vigouroux J, Thibault JF. Isolation and partial characterization of feruloylated oligosaccharides from maize bran. Carbohydr Res. 1995;272(2):241–253. doi:10.1016/0008-6215(95)00053-v.
- Zhang M, Zhang B, Qian Q, Yu Y, Li R, Zhang J, Liu X, Zeng D, Li J, Zhou Y. Brittle Culm 12, a dual-targeting kinesin-4 protein, controls cell-cycle progression and wall properties in rice. Plant J. 2010;63(2):312–328. doi:10.1111/j.1365-313X.2010.04238.x.
- Wu B, Zhang B, Dai Y, Zhang L, Shang-Guan K, Peng Y, Zhou Y, Zhu Z. Brittle culm15 encodes a membrane-associated chitinase-like protein required for cellulose biosynthesis in rice. Plant Physiol. 2012;159(4):1440–1452. doi:10.1104/pp.112.195529.
- Schindelman G, Morikami A, Jung J, Baskin TI, Carpita NC, Derbyshire P, McCann MC, Benfey PN. COBRA encodes a putative GPI-anchored protein, which is polarly localized and necessary for oriented cell expansion in Arabidopsis. Genes Dev. 2001;15(9):1115–1127. doi:10.1101/gad.879101.
- Szyjanowicz PM, McKinnon I, Taylor NG, Gardiner J, Jarvis MC, Turner SR. The irregular xylem 2 mutant is an allele of korrigan that affects the secondary cell wall of Arabidopsis thaliana. Plant J. 2004;37(5):730–740. doi:10.1111/j.1365-313x.2003.02000.x.
- Sanchez-Rodriguez C, Bauer S, Hematy K, Saxe F, Ibanez AB, Vodermaier V, Konlechner C, Sampathkumar A, Ruggeberg M, Aichinger E, et al. Chitinase-like1/pom-pom1 and its homolog CTL2 are glucan-interacting proteins important for cellulose biosynthesis in Arabidopsis. Plant Cell. 2012;24(2):589–607. doi:10.1105/tpc.111.094672.
- Li F, Xie G, Huang J, Zhang R, Li Y, Zhang M, Wang Y, Li A, Li X, Xia T, et al. OsCESA9 conserved-site mutation leads to largely enhanced plant lodging resistance and biomass enzymatic saccharification by reducing cellulose DP and crystallinity in rice. Plant Biotechnol J. 2017;15(9):1093–1104. doi:10.1111/pbi.12700.
- Fujita M, Himmelspach R, Hocart CH, Williamson RE, Mansfield SD, Wasteneys GO. Cortical microtubules optimize cell-wall crystallinity to drive unidirectional growth in Arabidopsis. Plant J. 2011;66(6):915–928. doi:10.1111/j.1365-313X.2011.04552.x.
- Li F, Zhang M, Guo K, Hu Z, Zhang R, Feng Y, Yi X, Zou W, Wang L, Wu C, et al. High-level hemicellulosic arabinose predominately affects lignocellulose crystallinity for genetically enhancing both plant lodging resistance and biomass enzymatic digestibility in rice mutants. Plant Biotechnol J. 2015;13(4):514–525. doi:10.1111/pbi.12276.
- Zhang W, Yi Z, Huang J, Li F, Hao B, Li M, Hong S, Lv Y, Sun W, Ragauskas A, et al. Three lignocellulose features that distinctively affect biomass enzymatic digestibility under NaOH and H2SO4 pretreatments in Miscanthus. Bioresour Technol. 2013;130:30–37. doi:10.1016/j.biortech.2012.12.029.