?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
We aimed to determine whether humic acid (HA) can alleviate the injury of millet caused by drought and its potential mechanism. Millet seeds (Jingu 21 and Zhangza 10) were soaked in different concentrations of HA (0, 50, 10, 200, and 300 mg L−1) for 12 h. The physiological and photosynthetic characteristics of millet seedlings, including growth parameters, osmotic regulators, antioxidase activity, photosynthesis, chlorophyll fluorescence, and P700 parameters, were determined before and after drought stress. HA significantly promoted the growth of millet seedlings under drought stress. Pretreatment with 100 mg L−1 or 200 mg L−1 HA significantly increased free proline, soluble protein, and activity of the antioxidant enzyme system (superoxide dismutase, peroxidase, and catalase) in both Zhangza 10 and Jingu 21. The accumulation of reactive oxygen species ( and H2O2) was reduced in HA treatments compared with that of the control (P < .05). Moreover, HA (100 mg L−1) significantly increased net photosynthetic rate, stomatal conductance, effective quantum yield of photosystem II, relative photosynthetic electron transfer rate of photosystem II, and photochemical quenching. HA also reduced intercellular CO2 concentration and non-photochemical quenching. Furthermore, 200 mg L−1 HA significantly increased the maximum P700, effective quantum yield of photosystem I, and relative photosynthetic electron transfer rate of photosystem I in Zhangza 10 and decreased non-photochemical energy dissipation in Jingu 21 and Zhangza 10 under drought stress. HA promoted the growth of millet seedlings under drought stress by promoting the osmotic adjustment ability and antioxidant capacity of seedlings and increased photosynthesis.
Introduction
Drought stress is one of the most important abiotic stresses that adversely influences the growth and production of cropsCitation1. As a consequence of drought stress, both stomatal and non-stomatal limitations lead to a decline in photosynthesis.Citation2 A recent study showed that drought results in up to 21% and 40% yield reductions in wheat and maize, respectively.Citation3,Citation4 Currently, the shortage of water resources is becoming increasingly prominent with the advent of global warming.Citation5 Therefore, alleviating of drought resistance of crops has become a critical topic, which urgently needs to be solved.
Humic acid (HA) is a principal component of humic substances, which is present in various sources, such as soil, humus, peat, oxidized lignite, and coal.Citation6 HA can have various biochemical effects on plants, such as increasing cell membrane permeability, increasing photosynthesis and respiration rates, enhancing mineral uptake and enhancing protein synthesis and hormone-like activity.Citation7–Citation9 As a plant growth regulator, HA is also believed to play a significant role in improving drought tolerance.Citation10–Citation12 It has been reported that HA improves growth parameters, vegetative and generative yield, and the anthocyanin content of roselle (Hibiscus sabdariffa L.) under drought stress.Citation11 The application of glycine betaine and HA both as plant growth regulators have been shown to increase total dry matter, Pn (net photosynthetic rate), free proline content, soluble sugar content, and potassium content of Malus robusta seedlings under drought stress.Citation12 Gu et al. showed that HA can promote the growth of cucumber (Cucumis sativus L.) seedlings under nitrogen stress and increase the content of free proline and soluble protein in roots and leaves.Citation13 Studies have shown that HA can improve the osmotic adjustment and plasma membrane system of oat (Avena sativa L.) leaves under drought stress.Citation14 Furthermore, a study showed that the application of HA improves the Pn of rapeseed (Brassica napus L.) plants under drought stress via increasing the gas exchange rate and electron transport flux.Citation8 HA increases the chlorophyll content and Pn, and decreases the E (transpiration rate) of wheat under drought stress.Citation15 However, the mechanisms underlying HA-promoted drought tolerance, especially change in photosynthetic characteristics, are not completely understood.
Millet (Setaria italica (L.) Beauv.), first domesticated in China, is a staple food in the semiarid regions of east Asia.Citation16 According to Kuşvuran et al., HA treatments statistically significantly increased the yield and yield components of common millet (Panicum miliaceum L.).Citation17 Plenty studies have reported that drought stress decreased the millet yield.Citation18,Citation19 Currently, related studies on the effects of HA on millet under drought stress are limited. Whether the HA treatment can alleviate the drought stress on the millet? In this study, millet cultivars, Jingu 21 and Zhangza 10, were treated with different concentrations of HA. The effects of HA on the physiological and photosynthetic characteristics of millet seedlings under drought stress were evaluated, including the growth parameters, osmotic regulation, antioxidase activity (such as the superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT)), photosynthesis parameters, chlorophyll fluorescence parameters, and P700 parameters (Non-photochemical energy dissipation, Effective quantum yield of photosystem I, Quantum yield of non-photochemical energy dissipation, and Relative photosynthetic electron transfer rate of photosystem I). Our results may lay a foundation for the application of HA in millet.
Materials and methods
Plants and treatments
Jingu 21 and Zhangza 10 are the main varieties of millet in Shanxi Province. Jingu 21 has good quality in nutrition and palatable, poor resistance in environmental stress, and low yield. Furthermore, Zhangza 10 has strong resistance in environmental stress and high yield, but its quality in nutrition and palatable is lower than that of Jingu21. The seeds of millet cultivars, Jingu 21 and Zhangza 10, were provided by the Economic Crops Research Institute of Shanxi Academy of Agricultural Sciences and Zhangjiakou Academy of Agricultural Sciences, respectively. Plants were cultivated in the test fields of Shanxi Agricultural University under natural growing conditions. The potting soil (Pindstrup Mosebrug, Ltd., Denmark) contained more than 98% sphagnum moss and less than 2% impurities.
Treatments
Millet seeds were soaked with different concentrations of single compound HA (C9H9NO6, 227.17, dissolved in distilled water) (Shandong Xiya Chemical Industry Co., Ltd., Shandong, China) for 12 h (T0, 0 mg L−1; T1, 50 mg L−1; T2, 100 mg L−1; T3, 200 mg L−1; T4, 300 mg L−1). Millet seeds soaked with clean water were used as the control (CK). After natural drying on filter papers, the seeds were randomly and evenly seeded in nutrition pots (13 cm × 15 cm). In this study, we set up 3 replicates for each treatment and seeded 30 seeds for each repeat. Finally, three plants with similar growth states were selected as research objects. Because in the late stage of the experiment, we found that some plants may grow differently from most plants due to marginal effect and other reasons, so we excluded these plants. All the millet seedlings were cultivated in the open air and the plants were covered during raining. The seedlings of T and CK groups were artificially watering to ensure the normal growth of seedlings. The average temperature, humidity, photoperiod, and light intensity of the environment are listed in . When the seedlings grew to the 3–5 leaf stage (set as 0 d), watering was stopped for drought stress in the T0, T1, T2, T3, and T4 groups. However, the CK group continued to be normally watered. The substrate water content was measured using a soil moisture meter, TDR300 (Spectrum, USA). Based on the results of a preliminary experiment, the soil water content of the two millet varieties was the same after 5 and 10 d of drought stress. The water content at 5 and 10 d post-drought stress was 36.8% and 19.3%, respectively. The physiological parameters, growth changes, and photosynthetic characteristics of millet seedlings were measured at 5 and 10 d post-drought stress.
Table 1. The average temperature, humidity, photoperiod, and light intensity of the environment.
Measurements of seeding growth parameters
Plant height and stem diameter were measured using a tape measure and Vernier calipers, respectively. Leaf area was estimated based on the following formula: leaf area = length × width × 0.75, in which the length and width were the maximum length and width of the penultimate leaves of the seedling, respectively.Citation20 The aerial part of the plant was taken at the cotyledonary node and the fresh weight was measured. Then, the fresh plant tissue was dried in an oven at 105 °C for 30 min, followed by drying at 80 °C to a constant weight, and the dry weight was measured. Growth parameters were measured 10 d after drought treatment.
Measurements of seeding physiological parameters
The determination of relative water content (RWC) of leaves was performed on the penultimate leaves. The leaves were cut into pieces, and approximately 0.1 g of fresh leaf was immerged in distilled water for 12 h and the saturated weight was obtained. Then, the leaves were dried, and the dry weight was obtained. The RWC was calculated based on the following formula: RWC % = (fresh weight – dry weight)/(saturated weight – dry weight) × 100%. Leaf water potential was measured using a Psypro plant water potential meter (WESCOR, USA). The water potential of leaves was measured in situ using a leaf water potential probe. The Psypro water potential system could be connected by eight probes at a time. Data were collected sequentially from the first probe to the last probe, with a cycle time of 5 min. The chlorophyll content of the middle part of the penultimate leaves was measured by the spectrophotometer based on the method of Cui et al.Citation21 The chlorophyll content was measured by the following procedure. A 0.1 g sample from a penultimate leaf from the bottom of the seedlings was obtained and placed in a stoppered tube containing 10 mL of 96% ethanol. The tube was shaken to ensure that the samples were fully submerged in the solution. Then, they were maintained in the dark at room temperature for 24 h. Subsequently, the absorbance values of the supernatants were measured at 649 and 665 nm, respectively. The contents of chlorophyll a and chlorophyll b were calculated according to the following formulas: Chlorophyll a concentration (Ca) = 13.95 × OD665 – 6.88 × OD649; chlorophyll b concentration (Cb) = 24.96 × OD649 – 7.32 × OD665. We calculated total chlorophyll content as chlorophyll a + chlorophyll b.
Detection of free proline
The detection of free proline was performed by the acid ninhydrin method.Citation22 Briefly, 0.2 g of fresh leaves were placed in a mortar and ground with a small amount of quartz and 2.5 mL of 3% sulfosalicylic acid on ice. Grinding liquid volume was fixed to 5 mL and then placed in a boiling water bath for 10 min. After cooling, the grinding liquid was centrifuged at 1664 × g for 10 min, and the supernatant was obtained for further study. Approximately 2 mL supernatant, 2 mL glacial acetic acid and 2 mL acid ninhydrin were mixed and then placed in a boiling water bath for 30 min. After cooling to room temperature, 5 mL toluene was added to the mixture in the dark for extraction. The absorbance at 520 nm of the toluene phase (red) was measured by using 1 mL of distilled water and 1 mL of glacial acetic acid, and 1.5 mL of acid ninhydrin was used as a reference.
Detection of active oxygen related indexes
Superoxide anion () and hydrogen peroxide (H2O2) were detected using histochemical staining based on the procedures of Xu et al.Citation23 To determine these values, penultimate leaves were placed in a 50 mL centrifuge tube and stained with 0.5 mg mL−1 nitroblue tetrazolium (NBT) dye solution and 1 mg mL−1 diaminobenzidine (DAB) dye solution, respectively. After vacuum treatment, they were placed in the dark for 1 h and then the leaves were taken out and washed, followed by decolorizing with 50 mL of 96% ethanol for 24 h. Finally, the leaves were photographed to determine changes in
and H2O2. The production rate of
was detected by the hydroxylamine method.Citation24 The content of H2O2 was obtained based on the methods from Zhang et al.Citation25
Determination of antioxidant enzyme activity
Approximately 0.1 g of penultimate leaves, a small amount of quartz sand, and 2 mL of 50 mmol L−1 phosphate buffer (containing 0.1 mmol L−1 ethylenediaminetetraacetic acid and 1% polyvinylpyrrolidone) were mixed together and ground in an ice bath. Then, the mixture was centrifuged at 14972 × g for 15 min at 4 °C. The supernatant was used to determine the amounts of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and soluble proteins. The activities of SOD, POD, and CAT were detected by the nitroblue tetrazolium photoreduction method,Citation26 guaiacol colorimetric method,Citation22 and UV spectrophotometry,Citation26 respectively. In addition, soluble proteins were determined using Coomassie Brilliant Blue G-250 colorimetry.Citation27
Determinations of malondialdehyde (MDA) and relative electrolyte leakage
The content of MDA was measured by the thiobarbituric acid chromogenic method.Citation28 Briefly, 0.2 g of penultimate leaves, a small amount of quartz sand, and 2.5 mL of 0.1% trichloroacetic acid were mixed together and ground in an ice bath. Then, 5 mL 0.5% thiobarbituric acid was added to the mixture, followed by a boiling-water bath for 15 min, and was then centrifuged at 936 × g for 15 min after cooling to room temperature. The absorbance values of the supernatant at 532 nm and 600 nm were measured using 0.5% thiobarbituric acid as the control. The relative electrolyte leakage of the penultimate leaves was detected by a conductivity meter (DDS-11A, China). The 0.5 cm leaves were immersed in 10 mL of deionized water, followed by soaking for 12 h. Conductivity was measured as R1. After treating in a boiling water bath for 30 min and cooling to room temperature, the conductivity was measured as R2. The relative electrolyte leakage was calculated as R1/R2 × 100%.
Detection of photosynthetic parameters
In this study, the photosynthetic and fluorescence parameters were measured between 09:00 and 11:00. To ensure the consistency of the measurement time, three strains were determined for each treatment, and one leaf was examined for each strain and repeated three times. Photosynthetic parameters, including the transpiration (E), net photosynthetic rate (Pn), stomatal conductance (gs), and intercellular CO2 concentration (Ci) were detected in the middle of the penultimate leaves using a photosynthetic system (Li-6800, Li-COR, USA). The setting parameters were as follows: 90% red and 10% blue light, 800 μmol m−2 s−1 light intensity, and 400 μmol mol−1 CO2. According to the operating instructions of instrument, based on the CK, when the light intensity is 800, ΔF is between 1/3-2/3 of Fv.
Detection of chlorophyll fluorescence parameters
The chlorophyll fluorescence parameters were detected using a luminoscope (Dual-PAM-100, WALZ, Germany). After 30 min of dark adaptation, F0 was measured.Citation29 Then, the maximum fluorescence yield of the dark-adapted state (Fm) was detected under a saturated pulse (4000 μmol m−2 s−1). Followed by 40 s of darkness, 1 s of activated light (270 μmol m−2 s−1), and one saturated pulse, the maximal fluorescence yield of the light-adapted state (Fm′) was detected. Minimal fluorescence yield of the light-adapted state (F0′) was subsequently detected under far-red light (8 μmol m−2 s−1). The chlorophyll fluorescence parameters were calculated by the following formula:
Detection of P700 parameters
The P700 parameters were detected using a luminoscope (Dual-PAM-100, WALZ, Germany). After 30 min of dark adaptation, the initial P700 (P0) was detected under P700 detection light (18 μmol m−2 s−1). Then, maximum P700 (Pm) was detected under a saturated pulse light (4000 μmol m−2 s−1). Followed by 40 s of darkness, 1 s of activated light (270 µmol m−2 s−1), and one saturated pulse, the maximum P700 of the light-adapted state (Pm′) was detected. The P700 parameters were calculated by the following formula:
Statistical analysis was performed by SPSS version 16.0 (SPSS Inc., Chicago, IL). An analysis of variance (ANOVA) of the means of three replicates was performed. Quantitative data were expressed as mean ± standard error (SE), and multiple comparisons were analyzed using Duncan’s multiple range test. A p-value less than 0.05 was considered significantly different.
Results
Effects of HA on the growth parameters of millet seedlings under drought stress
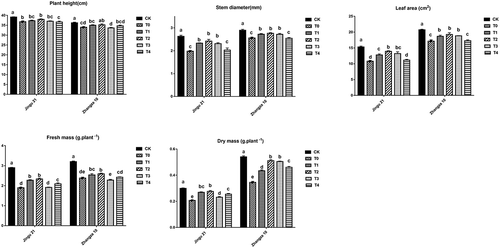
As shown in , drought stress (T0) significantly inhibited the growth of millet seedlings compared with that of the CK group. Under drought stress, with the increase in HA concentration, the growth parameters (plant height, stem diameter, leaf area, fresh mass, and dry mass) of the Jingu 21 and Zhangza 10 increased at first and then decreased. With the concentration of HA in T2 (100 mg L−1), the growth parameters of the two varieties reached the maximum. Comparison with the T0 group revealed that stem diameter, leaf area, fresh weight, and dry mass of Jingu 21 (T2 group) were significantly increased by 22.37%, 28.68%, 23.05%, and 33.33%, respectively. Likewise, plant height, stem diameter, leaf area, fresh weight, and dry mass of Zhang za 10 were significantly increased by 3.62%, 8.06%, 12.31%, 8.87%, and 48.12%, respectively, compared with that of the T0 treatment.
Figure 1. Effects of humic acid (HA) on the growth parameters of millet penultimate leaves under drought stress. CK, millet seeds soaked with clean water and watered normally; T0 T1, T2, T3, and T4 represented different concentrations of HA at 0 mg L−1, 50 mg L−1, 100 mg L−1, 200 mg L−1, and 300 mg L−1, respectively. Different lowercase letters in the same column indicate significant differences at P < .05.
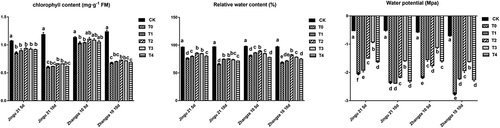
HA decreased the chlorophyll content, RWC, and water potential of millet seedlings under drought stress
As shown in , the chlorophyll content, RWC, and water potential of Jingu 21 and Zhangza 10 were significantly lower than those of CK after drought stress. Furthermore, the chlorophyll contents of the two varieties on the 5th d were lower than that on the 10th d after drought stress. When drought stress lasted for 10 d, the chlorophyll content of Jingu 21 and Zhangza 10 increased by 10.32% and 7.53% in the T2 group compared with that of T0 group, respectively. Moreover, HA significantly increased RWC and water potential of millet in the T2 and T3 groups compared with that of the T0 group (P < .05). Compared with that of T0, the water potentials of Jingu 21 in T3 after 5 and 10 d of drought stress increased 54.75 and 32.55%, respectively. The water potential of Zhangza 10 in T3 group was likewise increased by 48.69% and 41.41%, respectively.
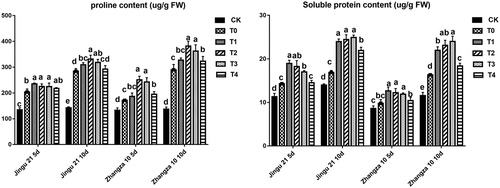
HA increased the free proline and soluble protein
As shown in , drought stress significantly increased the contents of soluble protein and free proline in Jingu 21 and Zhangza 10 (P < .05). Furthermore, the increase was positively correlated with the time of drought stress. Compared with that of the T0 group, the free proline level in the T2 group reached the maximum value when the drought stress lasted 10 d, increasing by 16.67% (Jingu 21) and 31.77% (Zhangza 10). The soluble protein content in the T3 group increased by 47.70% (Jingu 21) and 46.92% (Zhangza 10) comparing with that of the T0 group (drought stress lasting 10 d). The results suggested that under drought stress, the appropriate concentration of HA could promote the seedling to adjust the osmotic pressure-regulating substance, so as to resist the harm caused by drought stress.
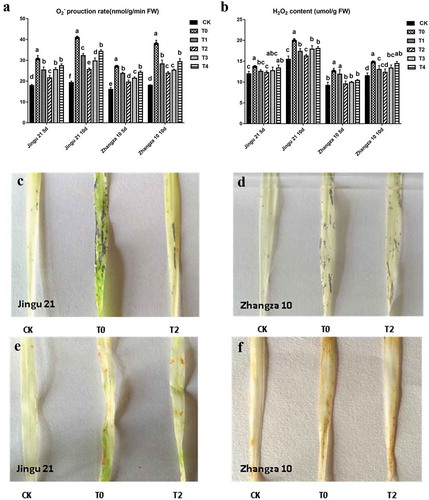
HA decreased the accumulation of active oxygen of millet seedlings under drought stress
As shown in a,b, under drought stress (T0), the production rates of and H2O2 in the two cultivars were significantly increased when compared with that of the CK, indicating that the accumulation of active oxygen was positively related to the length of drought stress. Furthermore, HA significantly reduced the production rate of
and H2O2 content of seedlings under drought stress. Histochemical staining for
(c,d) showed that there were no blue spots in the leaves of the CK group. Conversely, the leaves under a drought treatment showed a large area of blue, with the leaves in the T2 group showing scattered blue spots. The histochemical staining for H2O2 (e,f) showed that the brown portion of the T0 group was significantly greater than that of the T2 treatment.
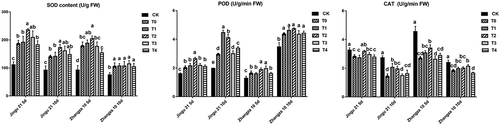
HA increased the activity of antioxidant enzymes under drought stress
As shown in , compared with that of the CK and T0 groups, the activities of SOD and POD increased significantly after drought stress both 5 d and 10 d (P < .05), whereas the activity of CAT decreased. After 5 d of drought stress, the content of SOD and POD of both cultivars showed a trend of increasing first and then decreasing with the increase of HA concentration, as well as after 10 d of drought stress. The results suggested that a suitable concentration of HA could increase the activity of antioxidant enzymes.
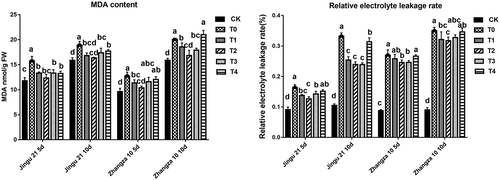
HA enhanced the MDA content and the relative electrolyte leakage of millet seedlings under drought stress
As can be seen from , compared with that of the CK, drought stress (T0) significantly increased the MDA content and relative electrolyte leakage of millet seedlings (P < .05). Furthermore, compared with that of the T0 group, the MDA content of Jingu 21 in the T3 and T2 groups was significantly lower by 21.68% (5 d) and 13.40% (10 d), respectively. In addition, the relative electrolyte leakage of Jingu 21 in the T2 group decreased by 22.76% (5 d) and 28.09% (10 d). The results suggested that a moderate amount of HA reduced the MDA content and relative electrolyte leakage of millet seedlings.
HA increased the Pn and gs and reduced the Ci of millet seedlings
As shown in , drought stress significantly reduced the Pn, E, and gs, and increased the Ci of Jingu 21 (10 d). For Zhangza 10, after 10 d of drought treatment, E, Pn, and gs all showed significant declines (P < .05). Both Jingu 21 and Zhangza 10 in the T2 group (100 mg L−1, 5 and 10 d) exhibited the highest Pn and gs under drought stress. After 5 d of drought stress, the Pn of Jingu 21 and Zhangza 10 in the T2 group was significantly higher by 54.53%, and 11.27% when compared with that of T0, respectively (P < .05).
Figure 7. Effects of humic acid (HA) on gas exchange parameters of millet penultimate leaves under drought stress. E, transpiration rate; Pn, net photosynthetic rate; gs, stomatal conductance; Ci, intercellular CO2 concentration.
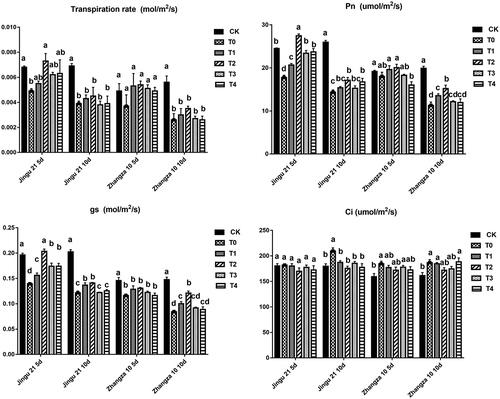
In addition, when the drought lasted for 5 d, the effect of HA in Jingu21 seedlings on Ci was not obvious. When compared with that of T0, the Ci of Jingu 21 in the T2 group was significantly decreased by 6.75%, and 16.23% under 5 and 10 d of drought stress, respectively (P < .05). Furthermore, different concentrations of HA did not significantly influence the E of Jingu 21 and Zhangza 10.
HA increased the Y(II), ETR(II), and qP, and reduced NPQ of millet seedlings under drought stress
As seen in , the effect of drought stress on Fv/Fm was not significant. Similarly, after 5 days of drought treatment, there was no significant change in Fv/F0 among different groups in both the two cultivars. With the extension of drought time, Fv/F0 of the two varieties showed different degrees of decline.
Figure 8. Effects of humic acid (HA) on chlorophyll fluorescence parameters of millet penultimate leaves under drought stress. Fv/Fm, maximal photochemical efficiency; Fv/F0, potential photochemical activity; Y(II), effective quantum yield of photosystem II; ETRII, relative photosynthetic electron transfer rate of photosystem II; NPQ, non-photochemical quenching; qP, photochemical quenching.
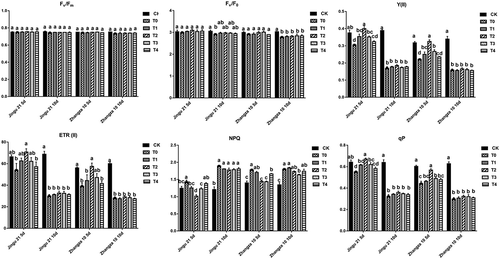
After 10 d of drought stress, compared with that of CK, all the other groups had significant reductions in Y(II), ETR(II), and qP, while increased in NPQ of Jingu 21 and Zhangza 10 seedlings. Both Jingu 21 and Zhangza 10 in the T2 group (100 mg L−1) exhibited the highest Y(II), ETR(II), and qP among different groups. Compared with that of T0, the Y(II), ETR(II), and qP of Jingu 21 in the T2 group was significantly increased by 31.15%, 31.06%, and 21.38% under 5 d of drought stress, respectively (P < .05). The Y(II), ETR(II), and qP of Zhangza 10 in the T2 group was significantly increased by 47.06%, 47.09%, and 28.51% under 5 d of drought stress, respectively (P < .05).
In addition, compared with that of T0, the NPQ of Jingu 21 in the T2 group was significantly decreased by 28.96%, and 6.17% under 5 and 10 d of drought stress, respectively (P < .05). The NPQ of Zhangza 10 in the T3 group was significantly decreased by 19.56% and 9.21% under 5 and 10 d of drought stress, respectively (P < .05).
HA increased the Pm, Y(I), and ETR(I), and reduced Y(NA) of millet seedlings
shows that after drought treatment, the Pm, Y(I), and ETR(I) of Zhangza 10 were significantly decreased compared with CK (P < .05). For Jingu 21, after 5 d of drought stress, the effect of various concentrations of HA on Pm, Y(I), and ETR(I) were irregular, while after 10 d, these indicators showed a significant decline compared with the control group (P < .05). Compared with that of T0, the Pm, Y(I), and ETR(I) of Jingu 21 in the T2 group were significantly increased by 30.71%, 19.87%, and 19.82% under 10 d of drought stress, respectively (P < .05). After 10 d of drought stress, the Y(NA) of both Jingu 21 and Zhangza 10 in the T3 group was significantly decreased by 14.52%, and 7.94% when compared with that of T0, respectively (P < .05).
Figure 9. Effects of humic acid (HA) on P700 parameters of millet penultimate leaves under drought stress. Pm, maximum P700; Y(i), effective quantum yield of photosystem I; ETR(I), relative photosynthetic electron transfer rate of photosystem I; Y(ND), quantum yield of non-photochemical energy dissipation; Y(NA), non-photochemical energy dissipation.
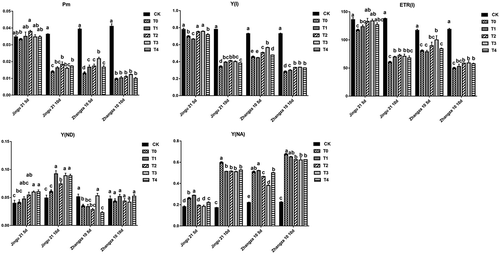
In addition, the Y(ND) contents of Jingu 21 were increased under drought stress, which was further enhanced with HA. However, different concentrations of HA did not significantly influence the Y(ND) of Jingu 21 and Zhangza 10. Zhangza 10 had higher drought resistance than Jingu 21
Discussion
Drought stress can cause an insufficient water supply to the plant and affect the normal growth of crops, which can lead to reduced yields. In this study, pretreatment of millet seeds (Zhangza 10 and Jingu 21) with HA increased chlorophyll content, RWC, and antioxidase activity of millet leaves under drought stress, which further improved the morphological indexes of plant height, stem diameter, leaf area, fresh weight, and dry weight of millet. This was particularly true for the dry weight of millet seedlings. The results indicated that the appropriate concentration of HA could effectively alleviate the damage of drought stress on millet seedlings.
The values of RWC and water potential reflect the ability to absorb water from the surrounding environment and are usually used to determine the drought resistance of plants.Citation30 Studies have reported that drought stress reduced the contents of chlorophyll, RWC, and water potential in plants, and the addition of specific exogenous substances could alleviate these symptoms.Citation31,Citation32 This study showed that HA showed similar functions. The results of our study were consistent with those of Elshabrawi et al., who showed that HA can promote wheat growth under newly reclaimed sandy soil with water deficiency.Citation33 The basic structure of humic acid is an aromatic ring, an alicyclic ring, and a hydrophilic group, such as a carboxyl group, a hydroxyl group, or a carbonyl group is bonded to enhance the water retention of the plant. Humic acid also has the effect of stabilizing the plasma membrane system by increasing the activity of antioxidant enzymes and participating in cell osmotic regulation. The osmotic adjustment of plants is the one of the main physiological mechanism for involved in the acclimation to drought stress. Under drought stress, plants accumulate a large amount of soluble protein and free proline to maintain a certain turgor pressure, which further prevents excessive water loss.Citation34,Citation35 Our study showed that drought stress increased the soluble proteins and free proline level of millet seedlings, whereas HA pretreatment further enhanced the soluble proteins and free proline level, which was similar to the finding of a study on rice by Muscolo et al.Citation36 The increased soluble proteins and free proline level could effectively prevent the cells from losing water. The results suggested that HA could improve the osmolytes under adverse conditions.
Drought stress triggers the production of , H2O2, ·OH, and other reactive oxygen species, which cause membrane lipid peroxidation. However, the antioxidant metabolism system, such as SOD, POD, and CAT, can effectively remove active oxygen and reduce membrane lipid peroxidation.Citation37,Citation38 Our study showed that drought stress promoted the accumulation of reactive oxygen species, such as
and H2O2 in millet seedlings. The 100 mg L−1 HA treatment significantly decreased the production rate of
and H2O2 content, and increased the activities of SOD, POD, and CAT of millet seedlings under drought stress, indicating that HA reduced the accumulation of reactive oxygen species caused by drought stress.
MDA is a product of cell membrane lipid peroxidation and relative electrolyte permeability is an index to measure cell membrane permeability. Both are important indicators of damage to the plasma membrane system. Campo et al. used MDA and relative electrolyte permeability as indicators to reflect the drought resistance of crops.Citation39 Studies have found that membrane lipid peroxidation caused by drought stress can cause MDA accumulation and cell membrane permeability damage, further leading to electrolyte extravasation and increased relative electrolyte permeability.Citation40,Citation41 Our study showed that under drought stress, MDA content and relative electrolyte permeability of millet seedlings increased significantly. The HA treatment significantly reduced the MDA content and relative electrolyte permeability of the seedlings and effectively alleviated the damage to the plasma membrane system of millet seedlings caused by drought. This is consistent with the results for rapeseed, wherein HA treatment significantly reduced the content of MDA in leaves and effectively alleviated the damage to cell membranes caused by water stress.Citation42
Inhibited photosynthesis is one of the main causes of crop failure under drought stress.Citation43 Because HA can enhance the photosynthesis of plants, HA is considered to be a potential treatment for the effects of drought stress in plants.Citation12,Citation44 In this study, the effects of HA on the photosynthetic characteristics of millet seedlings under drought stress were also evaluated. We found that HA, especially at a concentration of 100 mg L−1, significantly increased the chlorophyll content, RWC, Pn, and gs and reduced Ci of Jingu 21 and Zhangza 10 seedlings under drought stress. Furthermore, HA enhanced Pn was associated with increased Y(II), ETR(II), qP, Pm, Y(I), and ETR(I) and decreased NPQ and Y(NA) under drought stress.
In general, drought stress can reduce the chlorophyll content and gs of plants, thereby inhibiting the Pn.Citation45 The chlorophyll content is an important parameter in plants.Citation46 Studies have shown that the application of HA can increase the chlorophyll content of various plants, such as peppers,Citation47 grapes,Citation48 tomatoes,Citation49 and wheat.Citation50 In this study, we found that 100 mg L−1 HA significantly increased the chlorophyll content of Jingu 21 and Zhangza 10 under drought stress. Our findings are consistent with previous studies and further illustrated that HA can relieve the damage of drought stress on chlorophyll content. In addition, HA was also able to influence the Pn, gs, and Ci of plants.Citation51–Citation53 It has been reported that HA fertilizer significantly increases the Pn by 24.54% and gs by 25.93%, and reduced the Ci by 3.08% in tobacco.Citation51 HA treatments enhance the Pn and gs, as well as chlorophyll contents of Citrus reticulata Blanco.Citation52 The application of HA increases the gs and decreases the Ci of spring wheat at the flowering and fruiting growth stages.Citation53 Consistent with the above studies, we found that 100 mg L−1 HA significantly increased the Pn and gs of Jingu 21 and Zhangza 10 under drought stress. However, significantly decreased Ci induced by 100 mg L−1 HA was found in Jingu 21 but not in Zhangza 10. In the study of HA effects on photosynthesis, the most intuitive result was the prevention of chlorophyll loss, increased stomatal conductance, and increased photosynthetic rate. However, the fundamental effects of HA that alleviated drought symptoms for millet were to improve the water status of the seedlings. The RWC and water potential of the two varieties during drought for 5 and 10 d were significantly improved, effectively stabilizing the organelle structure, and maintaining cell growth and stomatal opening, thereby improving photosynthesis.
The mechanisms underlying HA-enhanced Pn are still not completely understood. In this study, the effects of HA on multi-subunit photosystem II were analyzed for the first time. We found that HA at a concentration of 100 mg L−1 significantly increased the Y(II), ETR(II), and qP of Jingu 21 and Zhangza 10, especially at an early drought stage (5 d). Furthermore, 100 and 200 mg L−1 HA significantly decreased the NPQ in Jingu 21 and Zhangza 10 under drought stress, respectively. Y(II), ETR(II), qP, and NPQ are important factors that respond to drought stress in photosystem II, which reflect the efficiency of light energy conversion, photosynthetic electron transfer, photosynthetic rate, and the dissipative light energy, respectively.Citation54 To stabilize the photosystem II center under drought stress, the utilization ability of light energy is reduced, electron transfer is slowed, and excess light energy is dissipated in the form of heat.Citation55 However, the application of HA reverses these reactions. Photosystem I is another important pigment system function in the photo-reduction of NADP+ in plants.Citation56 In Photosystem I, Pm, Y(I), and ETR(I) reflect the effective photosystem I complex, efficiency of light energy conversion, and photosynthetic electron transfer rate, respectively.Citation57 Under drought stress, the damage to photosystem I reduces the utilization ability of light energy and electron transfer.Citation58 In our study, with the prolongation of drought stress, the Pm of Jingu 21 and Zhangza 10 decreased by 4.03% (5 d) and 68.29% (10 d) and 59.65% (5 d) and 76.34% (d), respectively, compared with that of the CK, indicating that drought stress caused certain PSI damage. In the late stage of drought (10 d), Y(NA) of Jingu 21 and Zhangza 10 was increased by 206.95% and 196.19%, respectively, compared with that of the CK, indicating that the inactivation of key enzymes in the Calvin-Benson cycle caused electron accumulation in the PSI receptors. Y(I) and ETR(I) represent the photochemical rate and central electron transport rate of the PSI reaction center, respectively. At the late stage of drought stress (10 d), the significant decrease in Y(I) and ETR(I) for Jingu 21 and Zhangza 10 indicated that the actual photochemical rate and electron transport rate of PSI were decreased. However, we found that 100 and 200 mg L−1 HA significantly increased the Pm, Y(I), and ETR(I) in Jingu 21 and Zhangza 10 under drought stress. Furthermore, the Y(NA) of Jingu 21 and Zhangza 10 was significantly decreased by 200 mg L−1 HA under drought stress. The application of HA may alleviate the damage to photosystem I, thereby promoting the utilization of light energy and electron transfer. Furthermore, Y (NA) is an important index of light damage. The inactivation of key enzymes in the Calvin-Benson circulation can induce electron accumulation on the photosystem I acceptor side, thereby elevating Y(NA).Citation59 We suspect that HA may enhance Pn under drought stress through increasing Pm, Y(I), and ETR(I) and reducing Y(NA). In addition, our study showed that the leaf water balance ability, photosynthesis ability, and antioxidant capacity of Zhangza 10 were higher than that of Jingu 21. We conclude that the drought resistance of Zhangza 10 might be better than that of Jingu 21. The cause of this phenomenon is generally due to linkage drag.Citation60 In traditional breeding, good quality traits of crops are often accompanied by poor stress resistance through hybridization or back crossing.Citation61 In this study, HA was used to alleviate the damage of drought to millet, but the two varieties showed different degrees of mitigation. The deep reason may be caused by genetic factors.
In this study, we also found that, as shown in the –, with the increased of HA concentration, a number of physiological indicators of the two millet varieties showed a trend from gradual increase to gradual decline in mitigation of injury compared with CK, such as the fresh mass, dry mass, RWC, proline conten. Similar results were also found in the study by Zaremanesh et al. Under the same drought level, with the increase of HA concentration, the carotenoid, chlorophylla and total chlorophyll, RWC of purple coneflower (Echinacea purpurea) showed a trend of rising first and down later, or a trend down first and rising later.Citation62 A study has found that the relationship between the quality of fruit and the concentration of HA shows a significant secondary correlation. Therefore, appropriate concentrations should be taken when HA is applied in the field.Citation63
Our research had some limitation. First, The small sample size affects the accuracy of the results to some extent. Second, further large-scale field trials are also a important step in applying HA to millet. In conclusion, HA promotes the growth of millet seedlings under drought stress by promoting the osmotic adjustment ability and antioxidant capacity of the seedlings and increasing photosynthesis. Our findings may help to elucidate the mechanisms of the effects of HA on millet seedlings under drought stress and provide substantial guidance for the application of HA in agricultural production.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author contributions
Conception and design of the research: PG, YW; acquisition of data: JS, YW, SD; analysis and interpretation of data: XS, MG; statistical analysis: JS, MG; obtaining funding: PG, XY; drafting the manuscript: JS; revision of manuscript for important intellectual content: PG, YW. All authors read and approved the final manuscript.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Funding
References
- Chai Q, Gan Y, Zhao C, Xu HL, Waskom RM, Niu Y, Siddique KHM. Regulated deficit irrigation for crop production under drought stress. A review. Agron Sustainable Dev. 2016;36:1. doi:10.1007/s13593-015-0338-6.
- Gleason SM, Wiggans DR, Bliss CA, Comas LH, Cooper M, Dejonge KC, Young JS, Zhang H. Coordinated decline in photosynthesis and hydraulic conductance during drought stress in Zea mays. Flora. 2017;227:1–13. doi:10.1016/j.flora.2016.11.017.
- Saeidi M, Abdoli M. Effect of drought stress during grain filling on yield and its components, gas exchange variables, and some physiological traits of wheat cultivars. J Agric Sci Technol. 2018;17:885–898.
- Daryanto S, Wang L, Jacinthe PA. Global synthesis of drought effects on maize and wheat production. PLoS One. 2016;11:e0156362. doi:10.1371/journal.pone.0156362.
- Bavaru A, Bercu R. The global warming and the water resources of the earth. Diversity in Coastal Marine Science. Springer. 2018;23:79-88.
- El RF, Rouillon R, Vouvé F. Spectral characterization of the fluorescent components present in humic substances, fulvic acid and humic acid mixed with pure benzo(a)pyrene solution. Spectrochim Acta A Mol Biomol Spectrosc. 2018;199:71. doi:10.1016/j.saa.2018.03.030.
- Morozesk M, Bonomo MM, Souza IDC, Rocha LD, Duarte ID, Martins IO, Dobbss LB, Carneiro MT, Fernandes MN, Matsumoto ST. Effects of humic acids from landfill leachate on plants: an integrated approach using chemical, biochemical and cytogenetic analysis. Chemosphere. 2017;184:309. doi:10.1016/j.chemosphere.2017.06.007.
- Lotfi R, Kalaji HM, Valizadeh GR, Behrozyar EK, Hemati A, Gharavi-Kochebagh P, Ghassemi A. Effects of humic acid on photosynthetic efficiency of rapeseed plants growing under different watering conditions. Photosynthetica. 2017;56:1–9.
- Trevisan S, Francioso O, Quaggiotti S, Nardi S. Humic substances biological activity at the plant-soil interface: from environmental aspects to molecular factors. Plant Signal Behav. 2010;5:635–643. doi:10.4161/psb.5.6.11211.
- Sun T, Chen Y, Yang X, Hang B, Zhao G, Wang X. Effects of humic acid plant growth regulator on seed germination,growth and development. Humic Acid. 2012;4:19-25.
- Sanjarimijani M, Sirousmehr A, Fakheri B. The effects of drought stress and humic acid on morphological traits, yield and anthocyanin of roselle (Hibiscus sabdariffa L.). Agroecology. 2016;8:346–358.
- Zhang L, Gao M, Zhang L, Li B, Han M, Alva AK, ASHRAF M. Role of exogenous glycinebetaine and humic acid in mitigating drought stress-induced adverse effects in Malus robusta seedlings. Doga Turk J Bot. 2013;37:920–929. doi:10.3906/bot-1212-21.
- Gu XW D, Gao J. Effects of purified humic acid on growth and nitrogen metabolism of cucumber seedlings under nitrogen stress. J Appl Ecol. 2018;29:2575–2582. doi:10.13287/j.1001-9332.201808.029.
- Mishra B, Srivastava LL. Physiological properties of has isolated from major soil associations of Bihar. J Ind Soc Soil Sci. 1988;36:1–89
- Liu W, Liu J, Rula S, Hou G. Effect of humic acid water-soluble fertilizer on wheat photosynthetic characteristics and yield under water stress. Chin Agric Sci Bull. 2014;3:196–200.
- Mi J, Liu J, Zhang L, Chen X. Effects of humic acid on millet growth and soil water preservation under millet production with rainfed sandy soil in a semi-arid region. International Conference on Logistics Engineering, Management and Computer Science. 2015;7:1747–1753.
- VSA K, Babat S. The effect of different humic acid fertilization on yield and yield components performances of common millet (Panicum miliaceum L.). Sci Res Essays. 2011;6:663–669.
- Zhenhua W, Xin L, Aili Y, Kai C, Huixia L, Gang T. Effects of drought stress on dry matter accumulation in various organs and yield of millet at seedling stage. Guizhou Agric Sci. 2019;2:8–12.
- Lu H, Qiao Y, Gong X, Li H, Zhang Q, Zhao Z, Meng LL. Influence of drought stress on the photosynthetic characteristics and dry matter accumulation of hybrid millet. Photosynthetica. 2015;53:306–311. doi:10.1007/s11099-015-0120-7.
- Yu J, He Y, Zhao Z, Wang D. Study on the correction coefcient of crop area determined by long-width method. Jiangsu Agric Sci. 2007;4:37–39.
- Cui Q, Li X, Zhai S. Determination of chlorophyll content of wheat leaves by spectrophotometric method. J Anhui Agric Sci. 2006;34:2063.
- Zhang Z, Qu W. Plant physiology experiment. Beijing: higher education press; China. 2003.
- Xu J, Zhu Y, Ge Q, Li Y, Sun J, Zhang Y, Liu X. Comparative physiological responses of Solanum nigrum and Solanum torvum to cadmium stress. New Phytol. 2012;196(1):125–138. doi:10.1111/j.1469-8137.2012.04236.x.
- Elstner EF, Heupel A. Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem. 1976;70(2):616–620. doi:10.1016/0003-2697(76)90488-7.
- Zhang YP, Jia FF, Zhang XM, Qiao YX, Shi K, Zhou YH, Yu J-Q. Temperature effects on the reactive oxygen species formation and antioxidant defence in roots of two cucurbit species with contrasting root zone temperature optima. Acta Physiol Plant. 2012;34:713–720. doi:10.1007/s11738-011-0871-0.
- Li H. Plant biochemistry experiments and techniques. Educ Publishing Agency. 2000;195-7:258.
- Yongsheng LI, Fang Y, Yue LI, Ping MU, Wang F, Zhang T. Effects of exogenous hydrogen sulfide on seed germination and seedling growth under PEG stimulated drought stress in maize. J Nucl Agric Sci. 2016;4:813–821.
- Zou Q. Plant physiology experiment instruction. Beijing: China agriculture press; China. 2000.
- Guo M, Wang Y, Dong S, Wen Y, Song X, Guo P. Photochemical changes and oxidative damage in four foxtail millet varieties following exposure to sethoxydim. Photosynthetica. 2018;56:820–831. doi:10.1007/s11099-017-0734-z.
- Grzesiak MT, Grzesiak S, Skoczowski A. Changes of leaf water potential and gas exchange during and after drought in triticale and maize genotypes differing in drought tolerance. Photosynthetica. 2006;44:561–568. doi:10.1007/s11099-006-0072-z.
- Li Z, Tan X, Lu K, Zhang L, Long H, Lv J. Effects of drought stress on growth, gas exchange and chlorophyll fluorescence parameters of two species of tung oil. J Ecol. 2017;37:1515–1524.
- Bili C. Mechanism of silicon alleviation of drought stress in tomato (Solanum Lycopersicum L.). Shandong Agricultural University; 2015.
- Elshabrawi HM, Bakry BA, Ahmed MA, Abouellail M. Humic and oxalic acid stimulates grain yield and induces accumulation of plastidial carbohydrate metabolism enzymes in wheat grown under sandy soil conditions. Science. 2015;6:175–185.
- Subbarao VG, Chauhan SY, Johansen C. Patterns of osmotic adjustment in pigeonpea — its importance as a mechanism of drought resistance. Eur J Agron. 2000;12:239–249. doi:10.1016/S1161-0301(00)00050-2.
- Liu JH, Zhao HC, Ren YF, Zhang XQ, Wang Y. Change of osmotica in oat leaf under soil moisture stress. Acta Bot Boreali-Occidentalia Sin. 2009;7:1432–1436.
- Muscolo A, Sidari M, Attinà E, Francioso O, Tugnoli V, Nardi S. Biological activity of humic substances is related to their chemical structure. Soil Sci Soc Am J. 2007;71:75–85. doi:10.2136/sssaj2006.0055.
- Chen ZL, Li XM, Zhang LH. Effect of salicylic acid pretreatment on drought stress responses of zoysiagrass (Zoysia japonica). Russ J Plant Physiol. 2014;61:619–625. doi:10.1134/S1021443714050057.
- Yang LQ, Liu HR, Sun GW, Zhang SL, Dong LJ, Liu H, Jiang X, Liu J. Effects of drought stress on physiological property and growth parameter of different drought resistance soybean cultivars. J Agric Sci Technol. 2016;2:115–120.
- Campo S, Baldrich P, Messeguer J, Lalanne E, Coca M, San Segundo B. Overexpression of a calcium-dependent protein kinase confers salt and drought tolerance in rice by preventing membrane lipid peroxidation. Plant Physiol. 2014;165:688–704. doi:10.1104/pp.113.230268.
- Sun C, Johnson JM, Cai D, Sherameti I, Oelmüller R, Lou B. Piriformospora indica confers drought tolerance in Chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought-related genes and the plastid-localized CAS protein. J Plant Physiol. 2010;167:1009–1017. doi:10.1016/j.jplph.2010.02.013.
- Li-Ping B, Fang-Gong S, Ti-Da G, Zhao-Hui S, Yin-Yan L, Guang-Sheng Z. Effect of soil drought stress on leaf water status, membrane permeability and enzymatic antioxidant system of maize. Pedosphere. 2006;16:326–332. doi:10.1016/S1002-0160(06)60059-3.
- Lotfi R, Gharavi-Kouchebagh P, Khoshvaghti H. Biochemical and physiological responses of Brassica napus plants to humic acid under water stress. Russ J Plant Physiol. 2015;62:480–486. doi:10.1134/S1021443715040123.
- Rao DE, Chaitanya KV. Photosynthesis and antioxidative defense mechanisms in deciphering drought stress tolerance of crop plants. Biol Plant. 2016;60:1–18. doi:10.1007/s10535-016-0584-8.
- Zhang X, Ervin EH. Cytokinin-containing seaweed and humic acid extracts associated with creeping bentgrass leaf cytokinins and drought resistance. Crop Sci. 2004;44:1737–1745. doi:10.2135/cropsci2004.1737.
- Paknejad F, Nasri M, Moghadam HRT, Zahedi H. Effects of drought stress on chlorophyll fluorescence parameters, chlorophyll content and grain yield of wheat cultivars. J Biol Sci. 2007;7:841–747.
- Song T, Zihan HE, Cheng Y, Zhao R, Chunyan WU, Zhang X. Analysis on correlation between leaves SPAD value and chlorophyll content of flowering Chinese cabbage. J Northeast Agric Sci. 2017;6:273–295.
- Liu YF, Luo J, Tian-Ming SU, Fan RQ, Xin LU, Yan SH. Physico-chemical properties of a soilless substrate and growth of pepper influenced by exogenous humic acid. Jiangsu J Agric Sci. 2016;7:164–175.
- Ferrara G, Brunetti G. Influence of foliar applications of humic acids on yield and fruit quality of table grape cv. Italia. J Int Des Sci De La Vigne Et Du Vin. 2008;42:79–87.
- Kamari SS, Peyvast GH, Ghasemnezhad M. Effect of humic acid on growth and yield of tomato cv. sabela. 2013;4:153–169.
- Daur I. Comparative study of farm yard manure and humic acid in integration with inorganic-N on wheat (Triticum aestivum L.) growth and yield . Tarim Bilimleri Dergisi. 2013;19:170–177.
- Xiao Y, Jiang YZ, Di LI, Wang P. Effect of humic acid on photosynthetic characteristics of continuous cropping flue-cured tobacco. J Anhui Agric Sci. 2016;3:16–21.
- Ahmad S, Abbas T, Yaseen M, Anwar R, Khan MM, Bilal RM, Pervez MA. Effect of humic acid on some morpho-physiological and bio-chemical attributes of kinnow mandarin (Citrus reticulata Blanco). Ashs Conference, Islamic Republic of Pakistan. 2009.
- Hao S, Linhu LI, Yan S, Miao S, Kang SU, University H. Effect of different fertilization systems on spring wheat and phoyosynthesis characteristic and yield in hetao irrigation district. J Inner Mongolia Agric Univ. 2018;39:10–16.
- Lu Y. Identification and roles of photosystem II assembly, stability, and repair factors in arabidopsis. Front Plant Sci. 2016;7:168. doi:10.3389/fpls.2016.00168.
- Zhao L, Deng X, Shan L. The response mechanism of active oxygen species removing system to drought stress. Acta Bot Boreali-Occidentalia Sin. 2005;25:413–418.
- Yamori W, Shikanai T. Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annu Rev Plant Biol. 2016;67:81. doi:10.1146/annurev-arplant-043015-112002.
- Suga M, Qin X, Kuang T, Shen JR. Structure and energy transfer pathways of the plant photosystem I-LHCI supercomplex. Curr Opin Struct Biol. 2016;39:46–53. doi:10.1016/j.sbi.2016.04.004.
- Wang WH, Chen J, Liu TW, Han AD, Simon M, Dong XJ, Dong X-J, He J-X, Zheng H-L. Regulation of the calcium-sensing receptor in both stomatal movement and photosynthetic electron transport is crucial for water use efficiency and drought tolerance in Arabidopsis. J Exp Bot. 2014;65:223. doi:10.1093/jxb/ert362.
- Ci D, Song Y, Du Q, Tian M, Han S, Zhang D. Variation in genomic methylation in natural populations of Populus simonii is associated with leaf shape and photosynthetic traits. J Exp Bot. 2016;67:723. doi:10.1093/jxb/erv485.
- Wambugu P, Ndjiondjop MN, Furtado A, Henry R. Sequencing of bulks of segregants allows dissection of genetic control of amylose content in rice. Plant Biotechnol J. 2018;16:100–110. doi:10.1111/pbi.12752.
- Rey E, Abrouk M, Keeble‐Gagnère G, Karafiátová M, Vrána J, Balzergue S, Soubigou‐Taconnat L, Brunaud V, Martin‐Magniette ML, Endo TR, Bartoš J. Transcriptome reprogramming due to the introduction of a barley telosome into bread wheat affects more barley genes than wheat. Plant Biotechnol J. 2018;16:1767–1777. doi:10.1111/pbi.12913.
- Zaremanesh H, Eisvand H, Akbari N, Ismaili A, Feizian M. EFFECTS OF DIFFERENT HUMIC ACID AND SALINITY LEVELS ON SOME TRAITS OF KHUZESTANI SAVORY (SATUREJA KHUZISTANICA JAMZAD). Appl Ecol Environ Res. 2019;17:5409–5433. doi:10.15666/aeer/1703_54095433.
- L Z. study on the effect of humic acid fertilizer on several fruit trees and crops. Northwest A&F University; 2019.