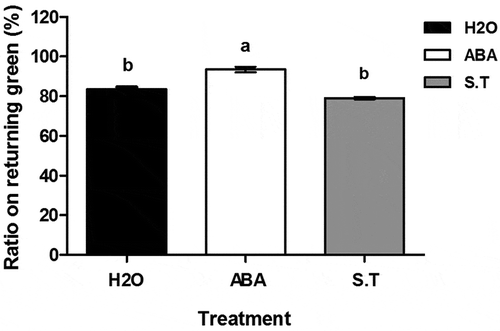
ABSTRACT
Abscisic acid (ABA) is an important plant hormone that plays significant roles in cold tolerance regulation. However, whether ABAimproves cold tolerance by increasing the activities of antioxidant enzymes in wheat remains unknown. In this study,the activities of antioxidant enzymes of the winter wheat variety ‘dongnongdongmai 1ʹ (‘dn1ʹ)afterthe application of exogenous ABA under low temperature (0°C, −10°C, −20°C, and −25°C) were investigated. Results showed that cold stress significantly increased H2O2 and relative conductivity, whileABA significantly reduced this effect. ABA enhanced cold tolerance in both leaves and rhizomes at −10°C and −20 °Cby increasing CAT, SOD, POD, APX, GR, DHAR, and MDHAR. However, this tolerance was weakenedat −25°C with decreasing ASA, GSH, APX, DHAR, and MDHARthan at-10°C and −20°C.POD, GR, and DHARlevels peaked at −10°C, while CAT, SOD, GSH, APX, and MDHAR content in rhizomes peaked at −20°C. The rate of returning green was significantly increased after ABA treatment than in controls (93.5% vs 83.6 %). In ‘dn1ʹ, rhizomes had a higher cold tolerance than leaves. Thereby, exogenous ABA could enhance cold tolerance byincreasing the activities of antioxidant enzymes.
Introduction
Low temperature stress is one of the adverseabiotic stressesthat plants frequently experience in their native growth environments.Citation1This stress severely limits plant growth and yield worldwide.Citation2 The dongnongdongmai1(‘dn1ʹ), cultivated by the Northeast Agricultural University, is the only winter wheat variety that can safelyoverwinter in the northern cold areas of China,such as theHeilongjiang province.The term “returning green” is defined as the recovery growth of a plant with leaves turning from yellow to green after winter. The returning green stage is a crucial periodwhen winter wheat encounters low temperature stress, and the returning green rate reflects the cold toleranceof a plant. Although the returning green rate of the ‘dn1ʹ is usually as high as 80–85%,it is dramatically decreased in a severe cold winter with less snow.Therefore, research to discover the mechanisms causingthe physiological changes and metabolic adjustments of ‘dn1ʹ in response to cold temperature are urgently needed.
Abiotic stresses typicallyinduce increased levels of reactive oxygen species (ROS), such as superoxide anion (O2·−) and hydrogen peroxide (H2O2),Citation3 which are toxic for the plants cells. Under normal conditions, ROS can beeffectively eliminated by non-enzymatic and enzymatic antioxidants (AOX), whereas under abiotic stresses, ROS is produced extensively and exceeds the capacity of the antioxidative systems to remove them.Citation4This cancause oxidative stress,which attacksthe membrane components,such as membrane lipids andmembrane proteins,and causes the peroxidation or oxidation of them.Citation5 To avoid these adverse effectsand to adaptto the environment, plants have to enhance the activity of antioxidant enzymes or increase antioxidant content. Accumulating evidenceshows that activities of several antioxidant enzymes, includingsuperoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR) are elevated under abiotic stresses such as water-, salt-, drought-, and cold stress.Citation6-Citation8 Abscisic acid (ABA) is an important plant hormone that plays significant roles in the regulation of osmotic stress tolerance. Endogenous ABA is often increased in response to drought, salt and cold stresses,Citation9 and the application of exogenous ABA is reported to significantly promote the activities of SOD, APX, CAT, GR, and GPX under cold stress inmaize seedlings.Citation10 These findings suggest that ABA might improve the cold tolerance of plantsby increasing the activities of antioxidant enzymes. Although the expression of several genes, such as the PeCPK10Citation11 and DREB1/CBF genes,Citation12in response to cold stressis related to ABA, expression of the corresponding enzyme genes are rarely reported.
This study aimsto investigate the changes in antioxidant enzyme activities and their corresponding gene expression inthe ‘dn1ʹwinter wheat variety in response to cold stress, and to explore theinfluence of the application of exogenous ABA on the cold tolerance.
Materials and methods
Plant materialsand ABA treatment
The experimental plant material used was ‘dn1ʹ,the winter wheat variety that was examined and approved by Heilongjiang province in 2007.As the returning green ratio of ‘dn1ʹ is more than 80%, it isidentified as the onlywinter wheat variety that can overwinter in the cold northern area of China.Seedswere mechanicallysowed in September 2010 in the experimental base of the Northeast Agricultural University in Heilongjiang Harbin (45° 34ʹ to 46ʹ N; 126° 22ʹ to 50ʹ E), whichis situated in the cold temperate continental climate.Black soil and cold are two famous characteristics of this area.In January, the average temperature is about −22°C,while the extreme temperaturerange is from-24°Cto – 32°C. The plantingbase was equally divided into different zones, each with the standard of 2 m × 0.5 m (row length × width). The spacing between zones was 0.5 m.
Three experimental treatments were conducted at the three-leaf stage (October 5, 2010, from5 PM to 6 PM). The leaves were sprayedwith 1 × 10-5mol/L ABA (ABA group);5 × 10-3mol/L sodium tungstate, the ABA inhibitors (S.T. group); and aclean water (control group), respectively, with the dosage rate of 1 L/row.Applicationwas conducted under good weather conditions with a breeze to ensure the quick absorption of these reagents.Each treatment was repeated 5 times.
Sample collection and determination methods
Leaf and rhizome sampleswere collected at four time points,October 15, 2010, November 30, 2010, December 22, 2010, and January 8, 2011;at which the minimum temperature had been stably maintained for at least 7 days at0°C, −10°C, −20°C, and −25°C, respectively. The samples were then stored at −80°Cin a refrigerator.
Relative conductivity is defined as the conductivity at a fixed time after freezing (R1) divided by the conductivity at a fixed time after high temperature sterilization (R2). It was calculated using the formula:(R1/R2)×100%.
H2O2activity was determined spectrophotometrically by detection at 410 nm as described by Jana and Choudhuri.Citation13 The activity of SOD was measured using the method proposed by Beyer and Fridovich.Citation14 Peroxidase (POD) activity was measured based on the determination of guaiacol oxidation at 470 nm by H2O2.Citation15 CAT activity was assayed from the rate of H2O2 decomposition following the method of Aebi.Citation16 APX activity was determined according to Nakano and AsadaCitation17at 290 nm. The GR activity was detectedby the formation of NADPH at 340 nm.Citation18 Dehydrogenation ascorbic acid reductase (DHAR) activity was assayed by following the detection of ascorbate (AsA) at 265 nm.Citation19 MDHAR activity was measured at 340 nm based on the method of Hossain et al.Citation20 Contents of AsA, DHA, and total ascorbate (AsA+DHA) were determined using the method of Hodgeset al.Citation21 Reduced glutathione (GSH) and oxidized glutathione (GSSG) were determined according to the method of Griffith.Citation22
Gene expression of the above-mentioned enzymes were detected using quantitative real-time reverse transcription-polymerase chain reaction (RT-qPCR). Total RNA was extracted using a Trizol kit (Sigma), and the corresponding primers were designed according to the homology sequences fromNCBI,as presented in .RNA was transcribed into cDNA using the GeneAmp® PCR system 9700 (Applied Biosystems, California, USA).Citation23Quantitative real-time RT-PCR was conducted on an Applied Biosystems’ ABI 7900 detection machine (Applied Biosystem, California, USA), with a 25μLreaction system consisting10 μL SYBR1 Green PCR Master Mix, 0.5μLof each primer, and 5 μL cDNA templates. The conditions for PCR were as follows: denaturation at 95ºC for 5 min, followed by 32 cycles of 95ºC for 15 s, 60ºCfor 45 s and 72ºC for 30 s, and a final extension of 72ºC for 10 min. The gene TaActin, which is constitutively expressed in wheat, was used as the internal reference. The relative amount of each transcript was determined using the 2−ΔΔCt method. Quantification was carried out using three biological replications.Citation24 The accession numbers of TaAPX, TaDHAR, TaMDHAR, and TaGR were FJ890988.1, AY074784.1, JX034702.1, and AJ010455.1, respectively.
Table 1. Primers for Real-Time RT-PCR* analysis.
The rate of returning green was calculated using the formula:
R = Na/Nb×100%
R: rate of returning green; Na: total number of the ‘dn1ʹ that returns green ‘dn1ʹ; Nb: total number of the ‘dn1ʹ.
Statistical analysis
Thedatafrommultiplegroupswerecompared by One-way ANOVA, followed by the post-hocleast significant difference (LSD)test, and significant difference was defined as P < .05.The Pearson correlation coefficient(PCC) was calculated to reveal relationships between genes.The variance analysis and correlation analysis was conducted using DPS 7.05software.
Results
Influence of exogenous ABA on H2O2 content and membrane damage of the‘dn1ʹvariety in response tolow temperature
Changes in H2O2 concentration
TheH2O2 contents in both rhizomes and leavesofthe ‘dn1ʹunder three treatments increasedwith decreasing ambient temperature. ().At the same temperature, H2O2 content in leaves() was slightly higher than in rhizomes().In leaves, ABA treatment significantly reduced the H2O2 content at 0°C and −20°C, compared to the control (P < .05); while there were no significant differences between the ABA treatment and the S.T. treatment plants at each temperature except 0°C (). In rhizomesthe H2O2 content was significantly decreased with ABA treatment compared with control plants, at all temperatures except 0°C (P < .05); and S.T. remarkably restored the reduction by ABA at −20°C and −25°C (P < .05) ().
Figure 1. Effect of exogenous ABA on H2O2 content and relative electrical conductivity of the ‘dn1ʹ variety.
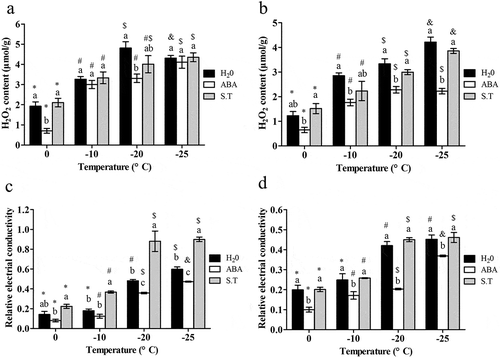
Changes in relative conductivity
With decreasing temperature, the relative conductivity in both rhizomes and leavesof the ‘dn1ʹincreased (). At the same temperature, the relative conductivity in rhizome () was lower in rhizomes than in leaves().In leaves, ABA treatment significantly reduced the relative conductivity compared to the control at −20°C and −25°C, and S.T. significantly restored the reduction of relative conductivity by the ABA group, even more than the control, at all the temperatures (P < .05) (). In rhizomes, the relative conductivity was significantly reduced with ABA treatment, and significantly increased, but not significantly more than the control, with S.T. treatment at all the temperatures(P < .05) ().
Influence of exogenous ABA on antioxidant enzyme activity of the ‘dn1ʹvariety in response to low temperature
Changes in SOD activity
In leaves, SOD activity in the control significantlyincreased with a decrease in temperature down to −20°C and −25°C, and SOD activity was highest at −20°C; ABA treatment significantly increased SOD activity (P < .05), while S.T. significantly reducedSOD activity at each temperature (P < .05)().In rhizomes, SOD activity gradually increasedin the controlwith decreasing temperature; ABA treatment caused a significant increase of SOD activity (P < .05), with the highest value at −20°Ccompared to the control, while S.T. significantly reduced the SOD activity at all temperatures(P < .05) (). SOD activity was higher in rhizomesthan in leaves at 0°Cand −10°C, butwas higher in leavesat −20°Cand −25°C.
Figure 2. Effect of exogenous ABA on activities of antioxidants of the ‘dn1ʹ variety.
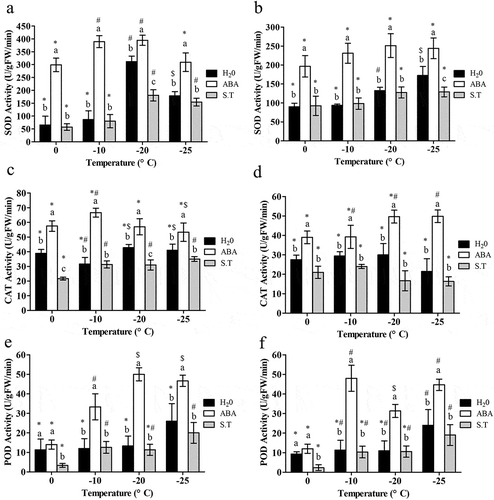
Changes in CAT activity
Changes of CAT activity in both rhizomes and leaveswereslightwhen temperature decreased ().Compared with the control, ABA treatment significantly increased the CAT activity in both leaves and rhizomesat each temperature (P < .05), and the CAT activity peaked at −10°C in leaves() and at −20°C in rhizomes (). S.T. treatment significantly reduced CAT activityin both leaves and rhizomes(P < .05) ().
Changes in POD activity
Astemperature decreased, the POD activity kept stable from 0 °C to −25°Cin leaves, but increased significantly at −25°Ccompared with 0°C in rhizomes(P < .05) ().In leaves, POD activity increasedsignificantly after ABA treatmentonce temperature decreased to −10°Cand peaked at −20°C.This increase was significantly inhibited by S.T. treatment at all temperatures(P < .05) (). In rhizomes, ABA also resulted in a pronounced increase of POD activity at all temperature points (P < .05), and POD peaked at −10°C. Likewise, S.T. significantly inhibited the increase in POD activity(P < .05) ().
Influence of Exogenous ABA on antioxidant content of the ‘dn1ʹvariety in response to low temperature
Changes in AsA activity
As temperature decreased,AsA contents in leaves slightlyincreased at first and then decreased and peaked at −10°C in leaves(),but no significant difference (), while AsA contents in rhizomewas only significantly increased at −20°C than in other ambient temperatures ().The AsA content in rhizomes was always less than in leaves at the same temperatures. In leaves, ABA treatment only significantly increased the AsA content at −20°C (P < .05), and S.T. significantly inhibited the increase at −10°C and −20°C (P < .05) (). However, neither ABA nor S.T. treatment had a significant influence on rhizome AsA activity at any temperature ().
Figure 3. Effect of exogenous ABA on contents of antioxidants of the ‘dn1ʹ variety.
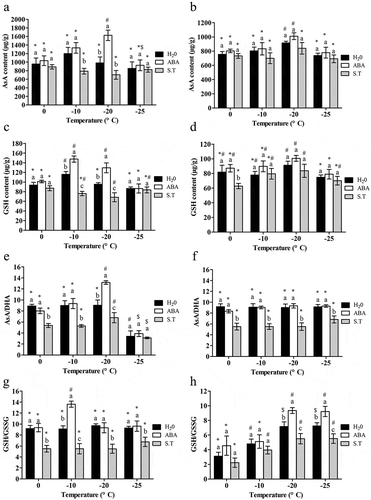
Changes in GSH activity
With decreasing temperatures, theGSH content increased in bothleaves and rhizomes at first and then decreased, with a peak at −10°C in leaves and at −20°C in rhizomes(). The GSH content in rhizomeswas lower in than leaves at the same temperature. In leaves, GSH content increasedsignificantly with ABA treatment(P < .05), and reduced significantly when treated with S.T. at −10°C and −20°C (P < .05) (). On the contrary, ABA did not cause any significant change in GSH activity in rhizomes, and asignificant reduction caused by S. T. treatment was only detected at 0°C (P < .05) ().
Changes of AsA/DHA and GSH/GSSG contents
The activityof AsA/DHA weregenerally not influenced bydecreasing temperature in both leaves and rhizomes, with the exception of at −25°C,wherethe values in leaveswere significantlylower(P < .05)().ABA treatment significantly increased the AsA/DHA content at −20°C in leaves(P < .05), and S.T. significantly decreased the content at all temperatures except −25°C (P < .05) (). In rhizomes, ABA treatment did not affect AsA/DHA activity at any temperature point, while a significant reduction was detected with S.T. treatment, compared with ABA treatment (P < .05)().
The contents of GSH/GSSG in rhizomesgradually increased () with decreasing temperature,but kept stablein leaves().ABA significantly increased the GSH/GSSG content at −10°C in leaves, and S.T. significantly decreased the content at all temperature points (). In rhizomes, ABA treatment significantly increased the GSH/GSSG contentat all temperatures (P < .05), while S.T. significantly decreased the GSH/GSSG content at −20°C and −25°C(P < .05) ().
Influence of exogenous ABAonAsA–GSH cycle-related enzyme activities andgene expression of the ‘dn1ʹ variety in response to low temperature
Changes inAPX activity and TaAPX expression
The activity of APX in leaves increased initially as temperature decreased, and the peaked at −10°C before decreasing somewhat; ABA treatmentreflected the same trend as the control, and significantly increased the activity at −10°C (P < .05); while S.T. significantly countered the increase by ABA at all temperatures except 0°C(P < .05) (). The change of expression pattern of TaAPXwas similar to the change of APX activity under all three treatments, except that ABA also caused a significant increase of TaAPXexpression at −25°C (P < .05) ().
Figure 4. Effect of exogenous ABA on APX and GR activity and TaAPXand TaGRexpression of the ‘dn1ʹ variety.
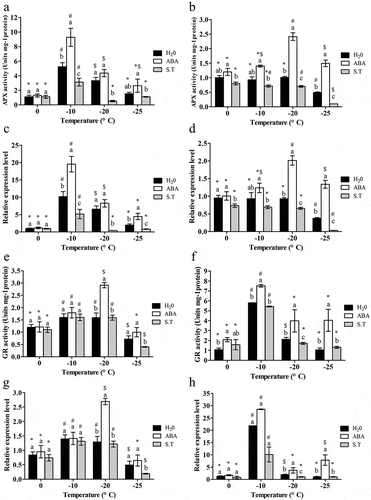
In rhizomes, both APX activity and TaAPX expression remained stable from 0°C to −20°C, and then significantly decreased at −25°C (). Trends ofAPX activity and TaAPX expression under ABA and S.T. treatments in the rhizomes were the same asin the leaves, except that the peak value was reached at −20°C ().
Changes in GR activity and TaGR expression
With decreasing of temperatures,the activity of GR in both rhizomes and leavesincreased at first and then decreased, withpeaks at-10 °Cand −20°C in leaves () and at −10°C in rhizomes ().In leaves, ABA only significantly increased GR activity at −20°C (P < .05), where the value peaked;while S.T. significantly decreased the activity at −20°C and −25°C(P < .05) (). In rhizomes, ABA caused a significant increase of GR activity at all temperatures (P < .05), and the activity peaked at −10°C; while S.T. significantly decreased GR activity except at 0°C (P < .05) ().
ChangesinTaGR expressionhad the same trend as GR activity under all three treatments in leaves and rhizomes ().ABA and S.T. had the same influence on GR activity and TaGR expression at all temperatures ().
Changes in DHAR activity and TaDHAR expression
As temperature decreased,the activity of DHAR in leavesincreased at first and then decreased, peaking at −10°C; ABA significantly increased the activity of DHAR at all the temperatures(P < .05) which peaked at −10°C; while S.T. dramatically reduced the activity of DHARat all the temperatures(P < .05)(). The expression trend of TaDHARwas the same as DHAR activity under all the treatments at all four temperature points ().
Figure 5. Effect of exogenous ABA on DHAR and MDHAR activity and TaDHAR and TaMDHARexpression of the ‘dn1ʹ variety.
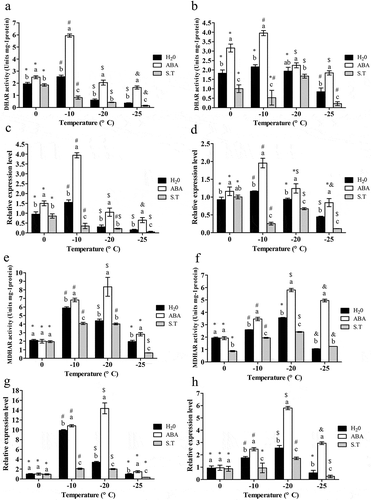
The patterns of both DHAR activity and TaDHAR expression were similar in both leaves and rhizomes, and both peaked at −10°C ().
Changes in MDHAR activity and TaMDHAR expression
In leaves,decreasing temperatures lead to increasingactivity of MDHAR at first, which then decreasedafter peaking at −10°C; ABA significantly increased the value of MDHAR except at 0°C (P < .05), and MDHAR peaked at −20°C; while S.T. had a significant decreasing effect on MDHAR except at 0°C (P < .05) ().Changes in TaMDHARexpression exhibited the same trend as MDHAR activity across all treatments at all temperatures ().
The effects of decreasing temperature on MDHAR activity and TaMDHAR expression were similar in rhizomes to that in leaves, except that the MDHAR activity peaked at −20°C () andTaMDHAR expression peaked at −20°C (). Likewise, ABA and S.T. had the same effects on changes of MDHAR activity and TaMDHAR expressionas in leaves ().
Correlation analysis among AsA-GSHcycle-related genes
The PCCsof gene expression of key metabolic enzymes in AsA-GSH cycle are listed in . Expression ofTaAPXwas significantly associated withTaMDHAR (r = 0.780, p < .01), and TaGRwas significantly associated with TaDHAR(r = 0.613, p < .05).
Table 2. Correlations of different gene expression in the AsA-GSH cycle.
Influence of exogenous ABAon returning green rate of the ‘dn1ʹ variety
Thereturning greenrate of the ‘dn1ʹvariety with ABA treatment was significantly higher thanin the control (93.5% vs 83.6%, P < .05) (), while S.T. treatment significantly decreased this ratecompared with the ABA treatment (79.1% vs 93.5%, P < .05).
Discussion
Under abiotic stress, levels of ROS (H2O2 and O2·−)are accumulated in plants.If ROS is not scavenged timely, it could cause the peroxidation of membrane lipids and the impairment of cellular membranes.Citation25 Therefore, it is understandable that the H2O2 contents and relative conductivity in leaves of the ‘dn1ʹ variety is significantly increased under cold stress. Antioxidant enzymes such as SOD, POD, CAT,and antioxidants in the AsA-GSH cycle play important roles in eliminating H2O2.Citation26,Citation27 SOD eliminatesO2·−and transforms it to H2O2,which is then transformedto H2Oby CAT.Citation28,Citation29 POD is also responsible for the elimination of H2O2 to protect plant tissue from lipid peroxidation.Citation30 Under abiotic stresses such as cold stress, enzymes in the AsA-GSH cycle are essential for the maintenance of cellular redox states and the detoxification of H2O2.Citation31Reportedly, SOD activity is elevated under cold stress in many plant species, such as chickpea and potato.Citation32,Citation33In Solanum melongena, activities of SOD, POD, and APX are significantly increased under cold stress.Citation34 In tobacco plants, elevated SOD and POD activities are found in leavesunder cold stress.Citation35In pepper seedlings, chilling stress significantly induces the AsA-GSH cycle.Citation36 The increased contents of AsAand GSH, and the increased activities of SOD, POD, CAT, APX, GR, DHAR and MDHAR could enhance the resistances of plants in various abiotic stresses.Citation37,Citation38In the present study, we found a unimodal bell-shaped trend ofthe activities of antioxidant enzymes (SOD, CAT and POD), contents of antioxidants (AsA, GSH, AsA/DHA, and GSH/GSSG), enzyme activity and AsA-GSH cycle-related gene expressionin leaves and rhizomes of the ‘dn1ʹ variety under cold stress. The activities of POD, GR, and DHAR peaked at −10°C, while thatof CAT, SOD, GSH, APX, and MDHAR in rhizomes peaked at −20°C. These results suggest that the ‘dn1ʹvariety hasa strong tolerance under cold stress caused by the increased activities of several antioxidant enzymes and their relevant gene expression.However, the tolerance was weakenedat temperatures below-25°C with decreasing ASA, GSH, APX, DHAR, and MDHAR activity reported compared to that at −10°C and −20°C.
ABA plays a crucial role in plant responses to cold stress. At normal growth temperatures, exogenous ABA could enhance the cold tolerance in herbaceous plants.Citation39 Under cold stress, exogenous ABA could also enhance the resistance of plants byenhancingthe antioxidant system. For instance, GSH and AsA levels and APX activity are higher in ABA-treated Stylosanthes guianensisSw. than those without ABA treatment.Citation40 By increasing the activities of SOD and PODand theexpression of Mn-SOD and POD, ABA is reported to enhance the tolerance to chilling-induced oxidative damage in peppers.Citation36 In the present study, we discovered that ABA had a remarkable effect in terms of reducing H2O2 contents and relative conductivity; while it had a significant influence on increasing the activities of antioxidant enzymes, levels of antioxidants, and AsA-GSH related enzyme activities and gene expression. However, the interplay between increasing activity in antioxidant enzymes and decreasing content of H2O2 was not obvious in the wheat leaves, which is possible because the accumulation of H2O2 was concentrated in the leaves during the removal process. Notably, ABA can significantly enhance antioxidant levelsat lower temperatures (from −10°C to −20°C), suggesting an improved cold tolerance of the ‘dn1ʹ variety. This might be the reason for the increased returning green rate of the ‘dn1ʹ with ABA treatment.
The responses to abiotic stresses might be different in leavesand rhizomes due to differences in cell specialization. Reportedly, 86% of the alterations caused by cold are different between rhizomes and leaves in Arabidopsis.Citation41 In support of this concept, we found that in rhizomes of the ‘dn1ʹ variety, change trends of several indicators were different than in leaves. For instance APX activity and TaAPX expression showed a unimodal bell-shaped response to decreasing temperature in leaves butstayed stable in rhizomes. Moreover, peak valuesin rhizomesoccurred at a lower temperaturefor AsA and GSH contents than in leaves (−20°C vs −10°C).In maize, the influence of ABA on leaves and roots under chilling-induced water stress are different: ABA content is highly associated with chilling tolerance at the leaf level but not at the root level.Citation42 In our study, ABA also showed a stronger effect on antioxidant enzyme activities and corresponding gene expression in rhizomesthan in leaves at each temperature. However, the peak value of APX activity and TaAPX expression were at a lower temperature in rhizomes than in leaves (−20°C vs −10°C). These results collectively suggest a higher cold tolerance of rhizomes than leavesof the ‘dn1ʹ variety.
APX in the AsA-GSH cycle can oxidize AsA to MDAsA, and MDHAR can restore MDAsA to produce AsA. The expression of TaGR is linked withTaDHARto maintain anappropriate redox level in plant cells. We found that expression of TaAPX and TaMDHAR were tightly associated, as was TaGR and TaDHAR, suggesting the consistent regulation of these genesby cold stress.
Although the effect of ABA on antioxidant enzymes or cold tolerance in wheat has been reported in past, how ABA can improve the cold tolerance by increasing the activities of antioxidant enzymes is rarely reported. In addition, our study has the following merits. First, our wheat cultivar (dn1) is an extreme cold resistant variety, which can resist freezing at −30°C, which is different from common winter wheat. Second, we tested the effect of different low temperatures (0°C, −10°C, −20°C, and −25°C) in open ground plots in order to reflect the long-term effects of lower temperatures with ABA treatment. Third, the detection indicators of antioxidant scavenging systems are now relatively complete. However, there are still several limitations of the present study. Many of the indicators reported here were not detected in other trials run for three years under similar environmental conditions, except for SOD, POD, and CAT. In addition, the roles of various isoforms of these enzymes and their correlated abundance with a low temperature response of wheat were not studied. Moreover, the enzymes we detected were not verified by other critical methods.
Conclusion
Exogenous ABA enhanced the cold tolerance in both leaves and rhizomes at −10°C and-20 °Cby increasing CAT, SOD, POD, APX, GR, DHAR, and MDHAR activity levels.The cold tolerance was weakenedat −25°C with decreasing ASA, GSH, APX, DHAR, and MDHARthan at temperaturesof-10°C and −20°C.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
Conception and design of the research: JC; acquisition of data: QL and BF; analysis and interpretation of data: QX and WL; statistical analysis: XW; obtaining funding: JC; drafting the manuscript: JY; revision of manuscript for important intellectual content: JC. All authors read and approved the final manuscript.
Data availability statement
All data generated or analyzed during this study are available upon request by contact with the corresponding author .
Additional information
Funding
References
- Oh S-J, Song SI, Kim YS, Jang H-J, Kim SY, Kim M, Kim Y-K, Nahm BH, Kim J-K. Arabidopsis CBF3/DREB1A and ABF3 in transgenic rice increased tolerance to abiotic stress without stunting growth. Plant Physiol. 2005;138(1):1–11. doi:10.1104/pp.104.059147.
- Tambussi E, Bartoli C, Guiamet J, Beltrano J, Araus J. Oxidative stress and photodamage at low temperatures in soybean (Glycine max L. Merr.) Leaves. Plant Science. 2004;167(1):19–26. doi:10.1016/j.plantsci.2004.02.018.
- Yusuf M, Hasan SA, Ali B, Hayat S, Fariduddin Q, Ahmad A. Effect of Salicylic Acid on Salinity-induced Changes in Brassica juncea. J Integr Plant Biol. 2008;50(9):1096–1102. doi:10.1111/j.1744-7909.2008.00697.x.
- Morales F, Abadía A, AbadÞa J. Photoinhibition and photoprotection under nutrient deficiencies, drought and salinity. In: Demmig-Adams B., Adams W.W., Mattoo A.K. (eds.), Photoprotection, photoinhibition, gene regulation, and environment. Springer, Dordrecht; 2008. p. 65–85.
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7(9):405–410. doi:10.1016/S1360-1385(02)02312-9.
- Nayyar H, Chander S. Protective effects of polyamines against oxidative stress induced by water and cold stress in chickpea. J Agron Crop Sci. 2004;190(5):355–365. doi:10.1111/j.1439-037X.2004.00106.x.
- Chang-Quan W, Rui-Chang L. Enhancement of superoxide dismutase activity in the leaves of white clover (Trifolium repens L.) in response to polyethylene glycol-induced water stress. Acta PhysiolPlantarum. 2008;30(6):841–847. doi:10.1007/s11738-008-0189-8.
- Srivastava AK, Bhargava P, Rai LC. Salinity and copper-induced oxidative damage and changes in the antioxidative defence systems of Anabaena doliolum. World J Microbiol Biotechnol. 2005;21:1291–1298. doi:10.1007/s11274-005-2442-2.
- Roychoudhury A, Basu S, Sengupta DN. Effects of exogenous abscisic acid on some physiological responses in a popular aromatic indica rice compared with those from two traditional non-aromatic indica rice cultivars. Acta PhysiolPlantarum. 2009;31(5):915–926. doi:10.1007/s11738-009-0305-4.
- Khorshidi M, Nojavan A. The effects of abscisic acid and CaCl2 on the activities of antioxidant enzymes under cold stress in maize seedlings in the dark. Pak J Biol Sci. 2006;9(1):54–59. doi:10.3923/pjbs.2006.54.59.
- Chen J, Xue B, Xia X, Yin W. A novel calcium-dependent protein kinase gene from Populus euphratica, confers both drought and cold stress tolerance. Biochem Biophys Res Commun. 2013;441(3):630–636. doi:10.1016/j.bbrc.2013.10.103.
- Kidokoro S, Watanabe K, Ohori T, Moriwaki T, Maruyama K, Mizoi J, Myint Phyu Sin Htwe N, Fujita Y, Sekita S, Shinozaki K. Soybean DREB1/CBF‐type transcription factors function in heat and drought as well as cold stress‐responsive gene expression. Plant J. 2015;81:505–518. doi:10.1111/tpj.12746.
- Jana S, Choudhuri MA. Glycolate metabolism of three submersed aquatic angiosperms during ageing. Aquat Bot. 1982;12:345–354. doi:10.1016/0304-3770(82)90026-2.
- Beyer WF, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. 1987;161(2):559–566. doi:10.1016/0003-2697(87)90489-1.
- Zheng X, Tian S. Effect of oxalic acid on control of postharvest browning of litchi fruit. Food Chem. 2006;96(4):519–523. doi:10.1016/j.foodchem.2005.02.049.
- Bergmeyer H-U. Methods of enzymatic analysis. Academic Press, New York—London; 1974;4.
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880.
- Esterbauer H, Grill D. Seasonal variation of glutathione and glutathione reductase in needles of picea abies. Plant Physiol. 1978;61(1):119–121. doi:10.1104/pp.61.1.119.
- Zhu Z, Wei G, Li J, Qian Q, Yu J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004;167(3):527–533. doi:10.1016/j.plantsci.2004.04.020.
- Hossain MA, Nakano Y, Asada K. Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 1984;25:385–395.
- Hodges DM, Andrews CJ, Johnson DA, Hamilton RI. Antioxidant compound responses to chilling stress in differentially sensitive inbred maize lines. Physiol Plant. 1996;98(4):685–692. doi:10.1111/j.1399-3054.1996.tb06672.x.
- Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106(1):207–212. doi:10.1016/0003-2697(80)90139-6.
- Zhu H-T, Dong Q-Z, Wang G, Zhou H-J, Ren N, Jia H-L, Ye Q-H, Qin L-X. Identification of suitable reference genes for qRT-PCR analysis of circulating microRNAs in hepatitis B virus-infected patients. Mol Biotechnol. 2012;50(1):49–56. doi:10.1007/s12033-011-9414-6.
- Zhang W, Jiang B, Guo Z, Sardet C, Zou B, Lam CS, Li J, He M, Lan H-Y, Pang R. Four-and-a-half LIM protein 2 promotes invasive potential and epithelial-mesenchymal transition in colon cancer. Carcinogenesis. 2010;31(7):1220–1229. doi:10.1093/carcin/bgq094.
- Boscolo A, Starr J, Sanchez V, Lunardi N, DiGruccio M, Ori C, Erisir A, Trimmer P, Bennett J, Jevtovic-Todorovic V, et al. The abolishment of anesthesia-induced cognitive impairment by timely protection of mitochondria in the developing rat brain: the importance of free oxygen radicals and mitochondrial integrity. Neurobiol Dis. 2012;45(3):1031–1041. doi:10.1016/j.nbd.2011.12.022.
- Liu Y-J, Yuan Y, Liu -Y-Y, Liu Y, Fu -J-J, Zheng J, Wang G-Y. Gene families of maize glutathione–ascorbate redox cycle respond differently to abiotic stresses. J Plant Physiol. 2012;169(2):183–192. doi:10.1016/j.jplph.2011.08.018.
- Shan C, Liang Z. Jasmonic acid regulates ascorbate and glutathione metabolism in Agropyron cristatum leaves under water stress. Plant Sci. 2010;178(2):130–139. doi:10.1016/j.plantsci.2009.11.002.
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48(12):909–930. doi:10.1016/j.plaphy.2010.08.016.
- Neill S, Desikan R, Hancock J. Hydrogen peroxide signalling. Curr Opin Plant Biol. 2002;5:388–395. doi:10.1016/S1369-5266(02)00282-0.
- Xu X-L, Zhang D-Y, Xu H-Y, Shen X-Y The Effects of Perfluorooctane Sulfonate (PFOS) on germination and physiological status of rice seedlings. Biomedical Engineering and Biotechnology (iCBEB), 2012 International Conference on: IEEE, Macao, China. 2012:1807–1810.
- Wang K, Shao X, Gong Y, Zhu Y, Wang H, Zhang X, Yu D, Yu F, Qiu Z, Lu H. The metabolism of soluble carbohydrates related to chilling injury in peach fruit exposed to cold stress. Postharvest Biol Technol. 2013;86:53–61. doi:10.1016/j.postharvbio.2013.06.020.
- Seppänen M, Fagerstedt K. The role of superoxide dismutase activity in response to cold acclimation in potato. Physiol Plant. 2000;108(3):279–285. doi:10.1034/j.1399-3054.2000.108003279.x.
- Kumar S, Kaur G, Nayyar H. Exogenous application of abscisic acid improves cold tolerance in chickpea (Cicer arietinum L.). J Agron Crop Sci. 2008;194:449–456.
- Wu X, He J, Zhu Z, Yang S, Zha D. Protection of photosynthesis and antioxidative system by 24-epibrassinolide in Solanum melongena under cold stress. Biol Plantarum. 2014;58(1):185–188. doi:10.1007/s10535-013-0377-2.
- Lin Y, Lin S, Guo H, Zhang Z, Chen X. Functional analysis of, a cytosolic glucose-6-phosphate dehydrogenase gene from, and its contribution to cold tolerance improvement in tobacco plants. Biotechnol Lett. 2013;9:1509–1518. doi:10.1007/s10529-013-1226-2.
- Guo W, Chen R, Gong Z, Yin Y, Ahmedand S, He Y. Exogenous abscisic acid increases antioxidant enzymes and related gene expression in pepper (Capsicum annuum) leaves subjected to chilling stress. Genet Mol Res. 2012;11(4):4063–4080. doi:10.4238/2012.September.10.5.
- Sultana S, Khew C-Y, Morshed MM, Namasivayam P, Napis S, Ho C-L. Overexpression of monodehydroascorbate reductase from a mangrove plant (AeMDHAR) confers salt tolerance on rice. J Plant Physiol. 2012;169(3):311–318. doi:10.1016/j.jplph.2011.09.004.
- Kornyeyev D, Logan BA, Payton PR, Allen RD, Holaday AS. Elevated chloroplastic glutathione reductase activities decrease chilling-induced photoinhibition by increasing rates of photochemistry, but not thermal energy dissipation, in transgenic cotton. Funct Plant Biol. 2003;30(1):101–110. doi:10.1071/FP02144.
- Xue-Xuan X, Hong-Bo S, Yuan-Yuan M, Gang X, Jun-Na S, Dong-Gang G, Cheng-Jiang R. Biotechnological implications from abscisic acid (ABA) roles in cold stress and leaf senescence as an important signal for improving plant sustainable survival under abiotic-stressed conditions. Crit Rev Biotechnol. 2010;30(3):222–230. doi:10.3109/07388551.2010.487186.
- Zhou B, Guo Z, Liu Z. Effects of abscisic acid on antioxidant systems of (Aublet) Sw. under chilling stress. Crop Sci. 2005;45(2):599–605. doi:10.2135/cropsci2005.0599.
- Kreps JA, Wu Y, Chang H-S, Zhu T, Wang X, Harper JF. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol. 2002;130(4):2129–2141. doi:10.1104/pp.008532.
- Aroca R, Vernieri P, Irigoyen JJ, Sánchez-Dı́ M, Tognoni F, Pardossi A. Involvement of abscisic acid in leaf and root of maize (Zea mays L.) in avoiding chilling-induced water stress. Plant Sci. 2003;165(3):671–679. doi:10.1016/S0168-9452(03)00257-7.