ABSTRACT
Rattan spines are most often regarded as an identification trait and perhaps as a physical protection structure. In this study, we study the spinescence traits from five different species rattan: Daemonorops lewisiana, Daemonorops geniculata, Calamus castaneus, Plectomia griffithii, and Korthalsia scortechinii. We tested length, width, angle, strength, spine density, cross-section surface, spine color, and leaf trichomes (only for D. lewisiana, C. castaneus and D. geniculata). We also tested whether the spines were capable of deterring small climbing mammals (for Plectomia griffithii and Calamus castaneus) by using a choice selection experiment. Due to a variety of spine traits, we could not categorize whether any species is more or less spinescent than the others. We suggest that spines have a much more significant role than merely as a physical defense and work together with other rattan characteristics. This is also evidenced by our choice selection experiment, in which the spines on a single stem donot deter small climbing mammals. However, this is a work in progress, and we have outlined several alternative methods to be used in future work.
Introduction
Plants, the most crucial producers in our ecosystem, are inevitably consumed by herbivores leading to evolutionary traits development to deter the herbivores. Toxic chemicals or repellents produced by many plants are considered as a chemical defense, whereas mechanical protection is based on the plant’s physical structures architecture to deter herbivores or decrease the efficiency of herbivory activities. Among all those protuberant structures, spines are one of the most prominent physical defensive traits in many plant species, and spines may act as the first barrier in deterring an array of herbivores.Citation1–Citation4 However, few studies were conducted on the structural defense in contrast to the considerable interest in studying plants’ chemical defense traits .Citation5
Spinescence describes the characteristics of plant protruding structures, spines thorns, and prickles. Spines are modified leaves with sharp-pointed stipule or petiole, while thorns come from modified branches, or twigs and prickles originated from cortical or epidermal tissue.Citation2,Citation6 Spinescence structures are deemed as a defensive weapon to deter herbivores since regional studies showed that well-defended plants correspond with the areas which are under heavy-browsing pressure. Citation7, Citation8, Citation54, Citation9–Citation12
Spinescence is not only associated with mammalian herbivores. For instance, New Zealand's plants possess similar spiny structures to deter large, avian herbivores, but spiny structures disappeared in many offshore islands where the avian herbivores did not co-exist.Citation13 The defensive abilities of spinescence also match with the ontogeny of the plant as some species deployed more spiny structures in their juvenile stage when heavy browsing happened, but they are less defended by such structures when they reach the mature stage.Citation13–Citation16 Research on the interaction between different kinds of herbivores and spiny plants showed that spines could decrease the biomass loss caused by herbivoresCitation17 and reducing the browsing efficiency of small climbing mammals .Citation18 Few studies demonstrated the relationships between spinescence and invertebrate herbivores e.g. Citation19 Therefore, spiny structures are more likely to offer deterrence against vertebrate herbivores rather than against invertebrate herbivores. However, studies also showed that in some instances, the protection provided by spines against some of the herbivores is not perfect.Citation20
Spinescence in Southeast Asian tropical rainforests is less studied. Rattan plants (subfamily Calamoideae) are common spiny palm species in Malaysia. There are around 600 species worldwide, and 110 species can be found in Peninsular Malaysia .Citation21-Citation23 Some of the most distinguishing features in rattan are the massive range of variety and arrangement of spiny structures on stems of various rattan species.Citation21 Since rattan may encounter different kinds of invertebrateCitation24 and vertebrate herbivores, Citation21 numerous spines on rattan are considered as a defensive weapon to deter herbivores .Citation21 There are numerous possible herbivores in the tropics, such as small climbing mammals (e.g tree shrews, rats, colugos), primates e.g(macaques, langurs) that might specifically target the rattan infructescence. However, the characteristics of spinescence are not well documented, much less the overall research interest in rattan itself.
In this study, we referred to the standardized measurement used by Citation6, and Citation55 on plant functional traits on five different rattan species (Daemonorops lewisiana, Daemonorops geniculata, Calamus castaneus, Plectomia griffithii and Korthalsia scortechinii). We propose a simplified three-point test in measuring the strength of the spine since the methods employed by Citation6 and Citation55 are hardly quantitative. We compared the different spiny structures among the five species to learn the defensive strength of each rattan species as well as the potential function of their different spiny arrangements. We also tested whether the spines (of Plectomia griffithii and Calamus castaneus) are effective in deterring small climbing mammals using a simple choice selection experiment.
Materials and method
Five different species of rattan (Daemonorops lewisiana, Daemonorops geniculata, Calamus castaneus, Plectomia griffithii, and Korthalsia scortechinii) were selected in this survey. The rattans were obtained from several different forests around Penang Island, Malaysia. For every species, five individuals were randomly sampled for their spines. Ten spines were randomly selected on each individual, and their length and width were measured using a vernier scale. The length of the spine was measured from the base to the tip of each spine. Width of spines was measured at the base part of each spine. Length and width were measured directly on the stem. Using a protractor, we measured the inclination of spines (10 from each plant). The density of spines was measured by estimating the number of spines on the densest part of each plant individual, which is usually the base part of the plant. Recurved spines on cirrus or flagellum stems were ignored since they serve as climbing organs.
To test the spine’s tensile strength, we used a generic digital pocket scale. To test the spine’s tensile strength, we used a generic digital pocket scale (1Set Pocket Electronic Digital Scale 10 G/40 kg). We purchased several units of the scale for testing the reliability of the measurement. The unique design of the spring system for this particular brand is that it allows measurement when the hook section is pushed in. We tested at first by attaching a fine thread to the middle of the spine and attaching it to the hook section, and we pulled the spring. We found that the readings for both pushing and pulling are similar. We decided to use the push method as it is more comfortable, compared to tying a piece of fine thread to a small spine. We tested several of the units, and the initial results were consistent and reliable. Ten spines ranging in length from 1.5 to 4.0 cm were randomly collected for tensile test. Clips were fixed to both sides of the spine. By pushing a digital weight scale in the middle part of each spine, the value will be recorded at the moment when the spines broke (). Reading was recorded in the unit gram from the digital scale, and it was converted to Newton [1 gram = 0.0098 Newton on earth).
Figure 1. The digital weight scale for tensile tests. We removed the original hook at the end of the metal tip.

As a clarification, we acknowledge that our suggested test is a simplified version of the three-point flexural test. Since our system is very basic, we could not calculate exact conditions such as fracture toughness or the stress intensity factor. We acknowledge that our test design did not factor in the diameter of the spines, which is quite a design problem since spines are elongated triangular. As for the distance between the two points, to achieve an accurate measurement, it must be standardized. This also presents a challenge in which some rattan spines are quite short. We adopted a simple approach of merely choosing the middle part of the spine, from tip to base. Due to the simplicity of our design, we could not calculate the displacement force. However, although our method is very robust, it does provide a simple quantitative value, and the test could be done in the field using fresh samples.
One stem of each species was taken out, and an image was taken from above to bottom to investigate their horizontal cut image. The horizontal cut image may provide a different viewing angle of herbivores’ perspective. We adopted the method used by Citation25. We took pictures of the spines, and the color of one spine from each species was measured using spectral measurement. The spectral measurement was performed using Ocean Optics Jazz spectrophotometer and analyzed by SpectraSuite software. Method of spectral measurement follows Citation16. The cross-section and the color measurement of the spines are essential as both have been hypothesized to have a deterrence effect on small climbing mammals if viewed from a climbing mammal perspective.
Leaf hairs/leaf trichomes can only be found on D. lewisiana, C. castaneus and D. geniculata. Length, angle, and the number of hairs on both sides and margin of leaflets from the three species were measured (]. This feature is not well documented in rattan, and their primary functions are not well studied.
Rattan spines as deterrence?
We tested several methods to test this, and we decided to use a modified choice-selection method from 18. We acknowledge that this portion of the results is a preliminary result from two species of rattans, which are Plectomia griffithii and Calamus castaneus. Comparison studies of the other species were still ongoing, and unfortunately, COVID 19 interrupted our field work. We were were not allowed to continue our project, and we have only the results from the two species of rattans that can be considered as complete. However, we feel that there is much information that we can derive for future studies based on the preliminary results of these two species. The experiment was conducted in a small patch of forest within USM main campus which allows easy access and monitoring by the researchers.
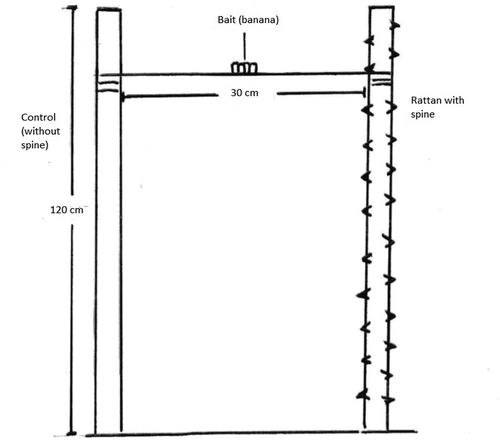
The rattan stem was set up vertically above the ground. The length used was standardized to 120 cm. We removed the spines on one of the stems (designated as the control). The length between the spine stem and control stem was set at 30 cm apart. A steel wire was connected between these stems, which will act as a platform for the small climbing mammals (). As an attractant to the animals, banana slices (3 slices) were put in the middle of the steel wire as bait. A M100 GameSpy Digital Camera was placed on the ground and angled at about 45° angle facing the rattan stems. The camera will sense any movement and automatically capture still images. The bait and camera were set up early in the morning (0600) and collected in the late evening (1800). The total observation was about 720 hours (2 months), with 360 hours per rattan species. We conducted the trials with Plectomia griffithii first and proceeded with Calamus castaneus in the subsequent month. We replaced the rattan stems with spines after a week, or if the spines deteriorated much earlier than that. We also alternated the position of the control and rattan spines every two days to prevent learning behavior. We also moved the experimental setup randomly several times within the forest. We were careful to choose a clearing which prevents any climbing mammals from jumping to the stems from nearby trees.
Result
The full results of the spinesence traits are listed in .
Table 1. The physical characteristics of the rattan spines. Alphabets in superscript denote significant differences for Tukey PostHoc analysis.
Cross-section surface
Cross-section images of D. lewisiana, D. geniculata, C. castaneus, P. griffithii and K. scortechinii are shown in –.
Specifically, for rattan D. geniculata (), spine length on the right side (72.03 ± 136 mm) of the is significantly different from the spine length on the left side (2432 ± 588 mm), t (12) = 10.22, p = .000. The angle of spines from the right side (123.0 ± 17.5°) is significantly larger than the angle of spines from the left side (87.40 ± 6.02°), t (4) = 4.29, p = .013.
Spine color
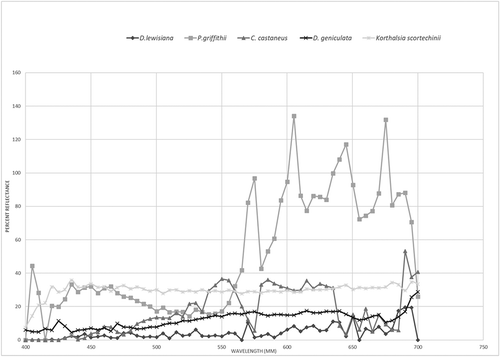
Spines in D. lewisiana are black with a yellow base (). Spines in D. geniculata are usually yellow to brown (). Spines in C. castaneus are generally bright yellow, but some individual spines are brown and black (). Spines in P. griffithii are green to yellow, and some are brown (). Spines in K. scortechinii are close to stem skins, which are green to yellow with a black tip (). The spectral reflectance results from 400 nm to 700 nm wavelengths are shown in .
Leaflet trichomes
Only three species (D. lewisiana, C. castaneus and D. geniculata) have small trichomes on both the abaxial and adaxial leaf sides, as well as on both margins of every leaflet. The arrangement of trichomes on leaflets differs. Daemonorops lewisiana’s leaflets have three rows of trichomes on the adaxial side but only one on the abaxial one. In C. castaneus and D. geniculata, however, the leaflet’s trichomes are arranged in an opposite way, three rows on the abaxial, and one row on the adaxial side of every leaflet. The margins are full of trichomes in all three species’ leaflets.
There were 34.8 ± 3.96 trichomes (4.01 ± 1.11 mm long) on the surface of D. lewisiana’s leaflets. The average distance between each trichome was 16.46 ± 6.42 mm. The average inclination of trichomes was 45.00 ± 9.13°, pointing to the tip of every leaflet. 78 ± 14.27 trichomes (1.90 ± 0.73 mm long) were found on the abaxial side of the leaflet, with an average distance of 3.68 ± 2.26 mm. Trichomes were pointing to the tip of the leaflet at 48.00 ± 8.88°. On the margins of leaflets, there were 81.80 ± 11.92 trichomes on one side of the leaflet (distance between each trichome 4.63 ± 1.95 mm) with the length of 1.61 ± 0.35 mm and 2.5 ± 6.43° of inclination toward the tip of the leaflet.
In C. castaneus, in the adaxial side, 15.4 ± 4.39 trichomes with an average length of 2.55 ± 0.72 mm and average inclination of 28 ± 5.37° pointing to the tip of every leaflet were found. In the abaxial side of every leaflet, 61.2 ± 13.44 trichomes 3.91 ± 0.85 mm long with an average distance of 14.37 ± 6.92 mm between each trichome and with an inclination of 56 ± 8.43° pointing to the tip of every leaflet were found. On the leaflet margins, most of the trichomes were very short (less than 1 mm) and clung to the margin (angle smaller than 10°). The average distance of every trichome was 4.81 ± 2.03 mm.
In D. geniculata, 6.4 ± 2.07 trichomes on the adaxial side with an average length of 2.19 ± 0.59 mm were found. Each trichome had an average distance of 25.80 ± 26.17 mm. The average angles of trichomes pointing to leaflet’s tip were 22.5 ± 4.56°. On the abaxial side of the leaflet, 51.4 ± 19.42 trichomes had an average length of 2.60 ± 0.75 mm and an average length 8.5 ± 4.46 mm between each trichome. Trichomes were pointing to the same direction (47.5 ± 10.61°). On the margin of every leaflet, 35.0 ± 6.86 trichomes had an average distance of 8.2 ± 3.69 mm. However, the length of most trichomes was short (less than 1 mm) and clung to the margin tightly (angle smaller than 10°).
Rattan spines as deterrence
During the experiment, we recorded only one type of small mammal that was consistently climbing the rattan stems. There were a few birds that landed on the wire and ate the bait, but we disregarded these as the birds are not climbers. We simply replaced the bait and moved the experiment setup to another place. The constant visitor was a common tree shrew (Tupaia glis). We cannot be sure how many individuals that climbed the stems but based on the difference in color and size, it was more than three individuals ().
Figure 15. Images captured by the camera shows that the Tupaia glis climbing the rattan spines. Figure A and C shows the tree shrew using the spines of C. castaneus as some sort of a climbing foothold, while figure B shows the tree shrew using the control spine. In figure D, the tree shrew used the control spine of P. griffithii to climb up to the bait.

The overall result showed that there was no significant difference in the selection between the control stem and the rattan spines for P. griffithii and C. castaneus χ2(1, N = 81) = 0.0024, p = .961. There were more attempts on the P. griffithii (N = 65) compared to the C. castaneus (N = 16).
Discussion
From the measurements of different aspects of stem spines from Fiverattan species as well as the features of leaf hairs from three rattan species, spines vary among the five species in different aspects. However, leaf hairs on the three species (D. lewisiana, C. castaneus and D. geniculata) were very similar to each other with a slightly different arrangement on both sides of the leaf surface. Measurements on spinescence are used to investigate the effectiveness of structural defenses against herbivores damages. Citation55 categorized five orders to indicate the defensive power of spinescence and the least defensive power is the plant with no spines while the most defensive power is the plant with the longest, with the highest density and the hardest spines that could harm herbivores or humans.
From our measurement of stem spines of the five rattan species, we could hardly categorize them from the least spinescent to the most spinescent. D. geniculata has the longest spines, and K. scortechinii has the shortest spines. The length of spines from D. lewisiana, C. castaneus and P. griffithii have no significant difference. Theoretically, the longer spine should be more effective in defending against herbivores compared to shorter spines. Hence, D. geniculata’s spines may be the most effective in deterring herbivores while K. scortechinii’s spines may be too short to be an effective defensive structure. Calamus castaneus has the widest spines while P. griffithii and K. scortechinii have the thinnest spines. With a similar length and a similar material, wider spines may be more stable than thinner spines. Therefore, it may be harder to remove those wider spines in C. castaneus compared to spines on other rattan species. However, it is tough to remove the very short spines on K. scortechinii from the stems.
The densest stem spines are found on C. castaneus, with more than 300 spines per 20 cm on the basal part of their stem, but the sparsest stem spines are on K. scortechinii, with not more than 100 spines per 20 cm on their stems. The other three rattan species have a similar density of spinescence. Studies have shown that longer spines and higher density can help plants reduce the efficiency of herbivory .Citation18,Citation26,Citation27 Quantitative data about spine density are scarce. Citation28 found that thorn density in two Mediterranean shrubs (Sarcopoterium spinosum and Calicotome villosa) that spread in areas suffering from high grazing pressure, range between 16,000 to 26,000 per m2 for S. spinosum, and between 6,000 and 11,000 per m2 for C. villosa. In the American cactus Opuntia ficus-indica grown as a spiny fence in Israel for centuries, spine density was found to be about 4,000 per m2 .Citation29 If the rattan stems were forming a dense matte, their spines were also at a density of several thousand spines per m2, a high spine density sufficient to provide good defense from mammalian herbivory.
However, the defense by spines may be influenced by other aspects. The orientation of the spines in rattans can be classified as two groups. K. scortechinii and P. griffithii have acute spine angles, which means that the majority of their spines were pointing downwards to the ground. The spines of the other three rattan species, D. geniculata, C. castaneus and D. lewisiana, have obtuse spine angle. Many of their spines were pointing upwards to the canopy. The down-pointing spine angles may be more effective in deterring small climbing mammals that consume the apical meristem from the bottom to top. The up-pointing spines were found to collect litter and debris, and this structure may help encourage colonization of defensive ants for protection from herbivory .Citation30
The strength of the spines is crucial in playing defensive roles as well. The stronger the spines, the tougher they are and more resilient toward herbivores. Daemonorops lewisiana has the strongest spine strength while spines in C. castaneus, P. griffithii and D. geniculata have weaker spines with little difference among each other. Hence, D. lewisiana’s solid spines may wound herbivores’ mouthpart or may be easier to penetrate herbivores’ thick skins compared to other species spines. The strength of the spines was not measured by previous studies on spine ecology, as it is not easy to quantify the strength of spines. It should be noted that thin spines might have a greater tendency to break into the herbivore’s tissues and cause infections than thicker and stronger ones.
The color of spines, which is conspicuous in the rattan species studied by us, can also contribute to deterring herbivores, especially for herbivores that forage by vision. Plant spines around the world display different defensive coloration.Citation3 For instance, in many African Acacia (recently classified as members of the genus Vachellia) species’ spines are of white color that makes them visually conspicuous .Citation31 In Pseudopanax crassfolius growing in New Zealand, conspicuously colored flanking spines exist only in juvenile leaves, but they are not formed in the adult stage, which matches the height that the plant could encounter herbivores.Citation16, Citation54 showed that for 167 wild species from Israel, spines, thorns and prickles, the typical colors are yellow, red, orange and white.
In this study, many spines of D. geniculata, D. lewisiana, and C. castaneus are black. The tip of spines on K. scortechinii are also black. Spines on P. griffithii are green to yellow. The conspicuous coloration was proposed to serve as aposematic [warning) coloration by Citation3, and it seems to serve the same defensive function as it does toxic or venomous animals’ warning decoration, which signals their enemies that the signalers are dangerous to be eaten. Therefore, aposematic coloration in spiny structures may display the defensive signals to herbivores that they are not palatable e.g. Citation3, Citation32. The black color on those spines could also serve as mimicry of ants. Black spots were also found on stems and branches of Xanthium trumarium (Asteraceae) by Citation33 and in other plant species Citation34). For instance, flowers of many Passiflora speciesCitation35 and petioles of Amorphophallus bufo (Ridl)Citation36 also express dark spots that seem to serve as visual ant mimicry. Numerous black spines on the stem of rattan species are also similar with a swarm of ants patrolling on their stem. Hence, herbivores could avoid plants that seem to be guarded by ants. From our field observations, many individuals of rattan from D. geniculate, D. lewisiana, C. castaneus and K. scortechinii were colonized by ants, but no ant colonies were found in P. griffithii, which matches with the presence of black spines on those four rattan species with ant colonies.
From the spiny stem structures of those five rattan species, K. scortechinii seems to have the weakest spiny structures since their spines are the shortest and the scarcest. However, they may have the most potent defensive ability against not only the vertebrate herbivores but also the invertebrate herbivores with the help of obligate ant mutualistic partners. Citation37 introduced a new concept as plant defensive syndromes, which describes that plants may develop a series of defensive strategies rather than a single trait to deter herbivores.
Rattan species may also possess other defensive strategies, such as increasing the hardness of leaves or the number of silica bodies (phytoliths) in plant tissues and increase the number of secondary compounds in plant tissues. Plants that cannot produce toxic chemical compounds can also deter herbivores by chemicals produced by symbiotic organisms such as bacteria or fungal endophytes .Citation38 Apart from defensive strategies, plants can also deploy avoidance strategies such as lowering the nutrient content in plant tissue or delay their growth to avoid herbivores in peak periods .Citation39 Plants with sufficient nutrients can grow more rapidly without defensive strategies, which are considered as tolerance strategies .Citation40
Rattan leaves are generally enormous, extending from the top of every stem. Spines on the stem can hardly prevent herbivores from eating their leaves. Leaf hairs on common mullein, Verbascum thapsu, are a defensive weapon against grasshoppers .Citation41 Leaf hairs on rattan species, D. geniculata, D. lewisiana, and C. castaneus, are arranged in different ways. However, they all have numerous hairs placed on the abaxial and adaxial leaf sides and both leaf margins. All the hairs in every leaflet are pointing to the tip of the leaflet, and the majority of the hairs accumulate at the distal leaflet part. They may discomfort herbivores’ mouthpart if herbivores eat leaves from the tip to the end. Leaf hairs may have multiple functions .Citation5
Although we do not find hairs on the rattan species P. griffithii and K. scortechinii, they may have alternative strategies to protect their leaves. Leaflets of K. scortechinii are praemorse, a term which describes the upper margins of the leaflet are erose or zigzag as if bitten offCitation21 (). That shape, giving the impression that herbivores ate the distal part of the leaflet, is well-known damage mimicry .Citation34,Citation42,Citation43 Similarly, Citation44 described a strategy as pseudo-variegation of leaves by displaying visual signals to pretend damages or occupation by herbivores may decrease the tendency of later attackers. Furthermore, rattan Korthalsia has close relationships with ant colonies and their leaves suffer less damage when patrolling ants protect the rattan plants .Citation45 During our survey, the ants occupying K. scortechinii were aggressive and displayed defensive behaviors, so the ants may help to protect rattan leaves. The leave protection of P. griffithii is poorly studied, and there is a layer of white powder at the back of every leaflet, which may act like trichomes for protection.
Spines are typically considered as induced plant response not only toward herbivoresCitation46 but also natural resistance, such as a fire .Citation47 Hence, in many plant species, the deployment of spiny structures may only happen when plants encountered herbivory or natural resistance. However, throughout this study, the same rattan species from different locations deployed similar patterns of stem spines. Stems of many rattan species are covered continuously by numerous spines, Citation21 and since rattans do not have secondary growth, their stem spines may not be inducible unless if damage may induce it on newly grown stems. The non-inducible feature of rattan stem may result from two factors. One may be the vital value of rattan stems in terms of surviving, and the other may be the alternative function of stem spines.
Palms (Family: Arecaceae) are a group of monocotyledons that only possesses one apical meristem. Leaves and flowers all rely on the growth of meristem and no secondary thickening for vascular bundles or external bark.Citation21,Citation48 Therefore, the single apical meristem, or the true apex which is called the edible “cabbage” by Citation21, is very vulnerable to the destruction from herbivores and the death of the single meristem will cause the death of the whole stem.Citation49 According to the Optimal Defense Hypothesis that defense should be contributed to the plant organs, structures of tissues which contain a high evolutionary value.Citation50,Citation51 Every apical meristem of the rattan plants is the indispensable part that when ruined, it is fatal to the individual stem. Hence, much defensive effort was contributed to stems so that a well-defended stem could maximize the fitness of every single plant. In many species, rattan spines on sheaths are always longer than the apical meristem.Citation21
Similarly, investigation of Brazilian palm species also showed no difference in stem spines with or without the presence of livestock.Citation9 Therefore, stem spines on rattan species may be a constitutive trait without much change through time and different locations. Rattan spines may also deploy various roles besides defense. According to Citation30, the rattans D. lewisiana and C. castaneus collect leaf litters from canopy level with the help of their up-pointing spines and the litter thus accumulated on their stem may form a nutrient trap and it also serves as a suitable environment for ant colonies. Ants on those rattans may enhance the nutrient content for the rattan and also protect the rattan plants from herbivores.
In our survey, D. geniculata’s spiny structures can also collect leaf litter. The longer spines on one side are always pointing up and form a closing environment around the main stem where much litter is collected and form a suitable environment for ant colonies. The spiny structures of D. geniculata are extraordinary as two distinct groups can be seen from their stem (, ). One group of spines are shorter with acute angles pointing to the ground while the spines of the other group are longer with obtuse angles pointing to the canopy level. The shorter spines may function as a defense since their acute spines could deter small climbing mammals from climbing up. The longer spines serve as collecting tools for litter so that the upward-pointing spines can trap falling leaves from the canopy level. Spines in the same plant may also serve as different functions by their growth patterns. Hence the multi-functioning spines on stems can still benefit the plant even no protective role is needed for deterring herbivores. The two reasons given above may explain why stem spines from different individuals, but the same species exhibited little differences.
Interestingly, our simple choice-selection experiment had shown that the rattan spines (of P. griffithii and C. castaneus) were not able to deter small climbing mammals. Based on the results of the selection of the rattan stem and there was no evidence of avoidance of the rattan spines. However, there were more attempts at climbing the P. griffithii compared to C. castaneus, although the sequential timing of the experiment might explain this. We conducted the P. griffithii first and the continued with C. castaneus in the next month. Attempts to climb the stems started to decline during the last week of using P. griffithii. Another possible reason is due to the difference in the rattan stem diameter itself, disregarding the spines. Rattan stems becomes narrower at the tip with a larger diameter at the base. Our experimental material acquirement is quite destructive as we need to cut the rattan stems every week, therefore to reduce damage, we began cutting and measuring from the tip, this would allow the rattan to regenerate. Therefore, the stems used during the last week (of both species) are large in diameter. We do acknowledge that this is hindsight on our part [that we did not standardize the diameter of the stem), but it does reveal an interesting hypothesis; what if the diameter of the rattan stem itself is an essential factor in deterring the climbing mammals? Citation52,showed that specific capabilities of small mammals are inherently linked to the diameter of the stem, but there is not an actual set pattern if whether smaller stems cause the small mammals to climb faster. Rather it depends entirely on the species preference. The tree shrews climbing preference in this study is not well documented, and we could only speculate. There is a caveat in this hypothesis from our results as there was no declining pattern for C. castaneus throughout the month. Therefore this question remains to be answered.
Our conclusion from this initial set of result is that the experimental design is not suitable to test the effectiveness of rattan spines to deter small climbing mammals. As outlined by the various traits of rattan spines [and even the hairs on the leaflet], the deterrence effect might work with the combination of several factors. A single spine stem might not be enough deterrence to thwart a hungry rodent. In natural conditions, the rattan stems are clumped together with a broad base at the bottom, and this might be the initial first deterrence toward the climbing mammals. 53 reported that for the infructescence of C. castaneus, Tupaia glis would examine the fruits, but did not eat or remove the fruits. The main fruit eater was Macaca nemesterina, which has the required dexterity to maneuver their hands to avoid the spines and pluck the fruits. We also had witnessed (in the field at the natural rattan habitat] how M. nemesterina would climb nearby trees (without spines) and with their acrobatic skill, would grab a flexible branch and using their body weight to lean in toward the rattan fruits. That way, the macaques could safely grab the rattan fruit. From this perspective, we can assume that using the banana as the bait might not be the best decision for the experiment. The shape and structure are different from a rattan fruit, which has overlapping scale layers. As such, we believe that 53 method of observing the rattan clump using video camera traps might be a better option. There is a downside to the original method as it is incredibly time-consuming since rattans fruiting period is quite unpredictable in the wild. We are currently pursuing this option with the additional tweak of manually removing the spines on one plant as the control. We expect to publish the results from this endeavor soon, as soon as we can begin our research after the COVID 19 lockdown.
In conclusion, the rattan spines are not exclusively designed for a physical defense only. There is no aggregating syndrome that can explain rattan spinescence on a general level. As shown by our deterrence experiment, a single stem of spines is not a practical design to deter a small climbing mammal. Each design of the spine is unique to each species, and there are other combinations of physical structures that work in tandem with the spines to be useful as a deterrent.
Acknowledgments
We thank the staff of the School of Biological Sciences and CEMACS for helping us with the sample collection. We also would like to thank Prof Simcha Lev-yadun and the two anonymous reviewers for improving this manuscript. We wish to thank Richard Simms for copyediting our manuscript. A special thanks go to Ms Zarith Soffy who helped with the camera trapping project. This project is funded under USM RUI grant PBIOLOGI/1001/8011124.
Additional information
Funding
References
- Cooper SM, Owen-Smith N. Effects of plant spinescence on large mammalian herbivores. Oecologia. 1986;68(3):1–16. doi:10.1007/BF01036753.
- Grubb PJ. A positive distrust in simplicity-lessons from plant defences and from competition among plants and among animals. J Ecol. 1992;80:585–610. doi:10.2307/2260852.
- Lev-Yadun S. Aposematic (warning) coloration associated with thorns in higher plants. J Theor Biol. 2001;210(3):385–388. doi:10.1006/jtbi.2001.2315.
- Supnick M. On the function of leaf spines in Ilex opaca. Bull Torrey Bot Club. 1983;110(2):228–230. doi:10.2307/2996348.
- Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM. Plant structural traits and their role in anti-herbivore defence. Perspect Plant Ecol Evol Syst. 2007;8(4):157–178. doi:10.1016/j.ppees.2007.01.001.
- Cornelissen JHC, Lavorel S, Garnier E, Diaz S, Buchmann N, Gurvich DE, Reich PB, Steege HT, Morgan HD, Van Der Heijden MGA, et al.A handbook of protocols for standardized and easy measurement of plant functional traits worldwide. Aust J Bot. 2003;51:335–380. doi:10.1071/BT02124.
- Burns KC. Are there general patterns in plant defence against megaherbivores? Biol J Linn Soc. 2013;111:38–48. doi:10.1111/bij.12181.
- Ford AT, Goheen JR, Otieno TO, Bidner L, Isbell LA, Palmer TM, Ward d, Woodroffe R, Pringle RM. Large carnivores make savanna tree communities less thorny. Science. 2014;346:346–349. doi:10.1126/science.1252753.
- Goldel B, Araujo AC, Kissling WD, Svenning JC. Impacts of large herbivores on spinescence and abundance of palms in the Pantanal, Brazil. Bot J Linn Soc. 2016;182:465–479. doi:10.1111/boj.12420.
- Milton SJ. Plant spinescence in arid southern Africa: does moisture mediate selection by mammals? Oecologia. 1991;87(2):279–287. doi:10.1007/BF00325267.
- Song B, Sun L, Lev-Yadun S, Moles AT, Zhang S, Jiang X, Gao Y, Xu Q, Sun H. Plants are more likely to be spiny at mid-elevations in the Qinghai-Tibetan Plateau in south-western China. J Biogeogr. 2020;47:250–260. doi:10.1111/jbi.13724.
- Takada M, Asada M, Miyashita T. Regional differences in the morphology of a shrub Damnacanthus indicus : an induced resistance to deer herbivory? Ecol Res. 2001;16(4):809–813. doi:10.1046/j.1440-1703.2001.00436.x.
- Burns KC. Spinescence in the New Zealand flora: parallels with Australia. N Z J Botan. 2016;54(2):273–289. doi:10.1080/0028825X.2015.1130727.
- Barton KE, Koricheva J. The ontogeny of plant defense and herbivory: characterizing general patterns using meta-analysis. Am Nat. 2010;175:481–493. doi:10.1086/650722.
- Brooks R, Owen-Smith N. Plant defences against mammalian herbivores: are juvenile Acacia more heavily defended than mature trees? Bothalia. 1994;24(2):211–215. doi:10.4102/abc.v24i2.773.
- Fadzly N, Jack C, Schaefer HM, Burns KC. Ontogenetic colour changes in an insular tree species: signalling to extinct browsing birds? New Phytol. 2009;184(2):495–501. doi:10.1111/j.1469-8137.2009.02926.x.
- Belovsky GE, Schmitz OJ, Slade JB, Dawson TJ. Effects of spines and thorns on Australian arid zone herbivores of different body masses. Oecologia. 1991;88:521–528. doi:10.1007/BF00317715.
- Cooper SM, Ginnett TF. Spines protect plants against browsing by small climbing mammals. Oecologia. 1998;113(2):219–221. doi:10.1007/s004420050371.
- Yamazaki K, Nakatani K-I, Masumoto K. Slug caterpillars of Parasa lepida (Cramer, 1799) (Lepidoptera: limacodidae) become stuck on rose prickles. Pan-Pac Entomol. 1914;90:221–225. doi:10.3956/2014-90.4.221.
- Potter DA, Kimmerer TW. Do holly leaf spines really deter herbivory? Oecologia. 1988;75(2):216–221. doi:10.1007/BF00378601.
- Dransfield J. A manual of the rattans of the Malay Peninsula. Kuala Lumpur (Malaysia): Forest Department, Ministry of Primary Industries Malaysia.; 1979.
- Dransfield J. The ecology and natural history of rattans. a guide to the cultivation of rattan. Forest Research Institute Malaysia. Kuala Lumpur. 1992;27–33.
- Dransfield J. General introduction to rattan-The biological background to exploitation and the history of rattan research. Rattan current research issues and prospects for conservation and sustainable development. Roma (Italia)Swedish International Development Cooperation Agency, Rome (Italy)International Network for Bamboo and Rattan, Beijing (Republic of ChinaFAO. 2002. 23–34.
- Xu HC, Rao AN, Zeng BS, Yin GT. Research on rattans in China: conservation, cultivation, distribution, ecology, growth, phenology, silviculture, systematic anatomy and tissue culture. 2000. IPGRI-APO, Serdang (Malaysia).
- Fadzly N, Mansor A, Zakaria R, Edzham SA. A view from a different angle: investigating the significance of rattan spines from a small mammals’ visual point of view using image. Sains Malaysiana. 2014;43:973–976.
- Gowda JH. Spines of Acacia tortilis: what do they defend and how? Oikos. John Wiley & Sons Ltd: UK; 1996. p. 279–284.
- Midgley JJ, Botha MA, Balfour D. Patterns of thorn length, density, type and colour in African Acacias. Afr J Range Forage Sci. 2001;18(1):59–61. doi:10.2989/10220110109485756.
- Ronel M, Malkiel H, Lev-Yadun S. Quantitative characterization of the thorn system of the common shrubs Sarcopoterium spinosum and Calicotome villosa. Isr J Plant Sci. 2007;55(1):63–72. doi:10.1560/IJPS.55.1.63.
- Nassar O, Lev-Yadun S. How prickly is a prickly pear? Isr J Plant Sci. 2009;57(1):117–124. doi:10.1560/IJPS.57.1-2.117.
- Liu K, Mansor A, Nadine R, Lee CY, Azman NM, Fadzly N. Rattan litter-collecting structures attract nest-building and defending ants. Plant Signal Behav. 2019;14:e1621245. doi:10.1080/15592324.2019.1621245.
- Midgley JJ. Why are spines of African Acacia species white? Afr J Range Forage Sci. 2004;21(3):211–212. doi:10.2989/10220110409485854.
- Lev-Yadun S. Aposematic (warning) coloration in plants. In: Baluska F. editor. Plant-Environment Interactions. Berlin (Heidelberg): Springer; 2009a.
- Lev-Yadun S, Inbar M. Defensive ant, aphid and caterpillar mimicry in plants? Biol J Linn Soc. 2002;77(3):393–398. doi:10.1046/j.1095-8312.2002.00132.x.
- Lev-Yadun, Simcha. Defensive (anti-herbivory) coloration in land plants. Cham: Springer International Publishing, Switzerland. 2016.
- Lev-Yadun S. Müllerian and Batesian mimicry rings of white-variegated aposematic spiny and thorny plants: a hypothesis. Isr J Plant Sci. 2009b;57(1):107–116. doi:10.1560/IJPS.57.1-2.107.
- Liu K, Fadzly N, Mansor A, Zakaria R, Ruppert N, Lee CY. The dual defensive strategy of Amorphophallus throughout its ontogeny. Plant Signal Behav. 2017;12(10):e1371890. doi:10.1080/15592324.2017.1371890.
- Agrawal AA, Fishbein M. Plant defense syndromes. Ecology. 2006;87:S132–S149.
- Clay K. Clavicipitaceous endophytes of grasses: their potential as biocontrol agents. Mycol Res. 1989;92(1):1–12. doi:10.1016/S0953-7562(89)80088-7.
- Coley PD, Barone JA. Herbivory and plant defenses in tropical forests. Annu Rev Ecol Syst. 1996;27(1):305–335. doi:10.1146/annurev.ecolsys.27.1.305.
- Rosenthal JP, Kotanen PM. Terrestrial plant tolerance to herbivory. Trends Ecol Evol. 1994;9(4):145–148. doi:10.1016/0169-5347(94)90180-5.
- Woodman RL, Fernandes GW. Differential mechanical defense: herbivory, evapotranspiration, and leaf-hairs. Oikos. UK: John Wiley & Sons Ltd; 1991. p. 11–19.
- Niemelä P, Tuomi J. Does the leaf morphology of some plants mimic caterpillar damage? Oikos 50, 256–257.
- Quicke DLJ. Mimicry, crypsis, masquerade and other adaptive resemblances. Wiley Blackwell; Oxford.
- Lev-Yadun S, Niemela P. Leaf pseudo-variegation: definition, common types, and probably the defended models for real defensive leaf variegation mimicking them? Flora. 2017;226:82–88. doi:10.1016/j.flora.2016.11.010.
- Miler K, Yahya BE, Czarnoleski M. Reduced damage and epiphyll cover of leaves of Korthalsia rattans that host Camponotus ants in the rain forest of Malaysian Borneo. J Trop Ecol. 2016;32(4):330–334. doi:10.1017/S0266467416000316.
- Karban R, Myers JH. Induced plant responses to herbivory. Annu Rev Ecol Syst. 1989;20(1):331–348. doi:10.1146/annurev.es.20.110189.001555.
- Gowda J, Raffaele E. Spine production is induced by fire: a natural experiment with three Berberis species. Acta Oecologica. 2004;26(3):239–245. doi:10.1016/j.actao.2004.08.001.
- Tomlinson PB. The structural biology of palms. Oxford University Press; 1990.
- Tomlinson PB, Horn JW, Fisher JB. The anatomy of palms: arecaceae-Palmae. UK: OUP Oxford; 2011.
- McKey D. Adaptive patterns in alkaloid physiology. Am Nat. 1974;108(961):305–320. doi:10.1086/282909.
- Stamp N. Out of the quagmire of plant defense hypotheses. Q Rev Biol. 2003;78(1):23–55. doi:10.1086/367580.
- Gallardo-Santis A, Simonetti JA, Vásquez RA. Influence of tree diameter on climbing ability of small mammalS. J Mammal. 2005;86(5):969–973. Oxford University Press (OUP). doi:10.1644/1545-1542(2005)86[969:iotdoc]2.0.co;2..
- Ruppert N, Mansor A, Anuar S. A key role of the southern pig-tailed macaque Macaca nemestrina (Linnaeus) in seed dispersal of non-climbing rattans in Peninsular Malaysia. Asian Primates J. 2014;4:42–51.
- Ronel M, Lev-Yadun S. The spiny, thorny and prickly plants in the flora of Israel. Bot J Linn Soc. 2012;168:344–352
- Peìrez-Harguindeguy N, Díaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P. New handbook for standardized measurement of plant functional traits worldwide. Aust J Bot. 2013;61:167–234