ABSTRACT
In order to find out the response mechanism of nitrogen assimilation and glutamine/glutamine family of amino acids metabolism in mulberry (Morus alba L.) leaves under NaCl and NaHCO3 stress, and to reveal its role in salt alkali adaptation. The effects of the nitrogen metabolism of mulberry leaves were studied under 100 mmol L−1 NaCl and NaHCO3 stress.The results showed that the activity of NR and the content of TN and SP did not change significantly, the expression of NiR, Fd-NiR, Fd-NiR gene and theactivity of NiR increased significantly under NaCl stress, but nitrogen assimilation was inhibited under NaHCO3 stress. NaCl stress had no significant effect on the expression and activity of GS and GOGAT in mulberry leaves. Under NaHCO3 stress, the expression of Fd-GOGAT, Fd-GOGAT2, Fd-GOGAT gene, and the activity of GS and GOGAT were significantly decreased. NaCl stress can promote the accumulation of Pro, Put and Spd in mulberry leaves. The accumulation of Pro under NaHCO3 stress is greater than that under NaCl stress. NaCl stress also induced the up-regulation of GAD, GAD1 and GAD1 gene expression, so promoting the synthesis of GABA may be an adaptive mechanism for mulberry to cope with NaCl stress, but the expression of GAD did not change significantly and GAD gene expression lower than CK under NaHCO3 stress. Although both NaCl and NaHCO3 stress could promote the synthesis of GSH by up-regulation of GCLM expression, GSH under NaHCO3 stress was significantly higher than that under NaCl stress, the content of H2O2 was still significantly higher than that of NaCl stress, that means GSH may not play a key role in alleviating the oxidative damage in mulberry leaves caused by salt and alkali.
Nitrogen is one of the most important factors limiting the growth of plants, and the yield and quality of crops.Citation1–Citation3 Nitrogen metabolism mainly consists of nitrogen uptake, transport, assimilation, etc. NO3–N and NH4+-N are the main forms of nitrogen used by plants. To be specific, NO3− can be assimilated as the glutamine/glutamine family and amino acid organic nitrogen by glutamine synthetase (GS) and glutamate synthase (GOGAT) only after being reduced into NH4+ under the actions of nitrate reductase (NR) and nitrite reductase (NiR).Citation4–Citation6 Amino acids synthesize proteins in cells, which are then modified, transported, and stored before becoming components of plant organisms. Reasonable nitrogen metabolism constitutes a precondition of normal plant growth. However, nitrogen metabolism is also a process relatively sensitive to saline-alkali adversity stress. Saline-alkali stress leads to the disorder of nitrogen uptake and metabolism in plants.Citation7 The amino acids of the glutamine/glutamine familymetabolites mainly include glutamate (Glu), glutamine (Gln), arginine (Arg) and proline (Pro), as well as γ-aminobutyric acid (GABA) and polyamines (PAs).Glu and Gln play vital roles not only in the nitrogen metabolism of plants, but also in the regulation of their carbon metabolism.Citation8,Citation9 Under abiotic stress, plants often accumulate Pro and PAs to regulate intra-cellular osmotic potential and promote water uptake. There is a positive correlation between the accumulation of Pro and the adaptability of plants to drought and salt stress ,Citation10 Pro also functions as signaling molecules in the growth and development of plants (such as inducing the expression of ABA) ,Citation11,Citation12 serves as an inducer for osmotic stress-related genes and a scavenger for reactive oxygen species (ROS) ,Citation13–Citation15 and plays a crucial part in regulating adversity stress in plants.Citation16 Under salt stress, the accumulation of Pro can also effectively reduce Na+ uptake by plant roots and lower Na+/K+ ratio in plants.Citation17,Citation18 The accumulation of PAs is a protective plant response to stress that enhances the resistance of plants to biotic and abiotic stresses.Citation19–Citation21 Besides osmotic regulation ,Citation22,Citation23 PAs are also capable of directly stabilizing cell membrane structures.Citation24,Citation25 GABA is also considered as a signaling molecule that is capable of regulating the growth and development of plants, and plays an important role in terms of improving the water retention capacity of cells ,Citation26,Citation27 regulating the transduction of cell signals ,Citation28 and adjusting the metabolism of ROS in plants, etc..Citation29,Citation30 The products generated in the metabolic process of the glutamine/glutamine family, such as GABA and NO, can also function as signaling molecules in regulating the growth and development of plants and their responses to adversities.Citation31–Citation33 In brief, the metabolic process of glutamine/glutamine family is not only affected by environmental factors, but also one of the most important mechanisms for plants to actively respond to adversities and improve their adaptability to adversities.
Mulberry(Morus alba L.) is a deciduous tree species of Moraceae. Besides being used as silkworm feed, its leaves also contain antioxidative active ingredients, which have important economical and medicinal values.Citation34,Citation35 In addition, mulberry is also resistant to drought, cold, and low temperature, and is a fine tree species with high economic and ecological values.Citation36,Citation37 More concretely, saline-alkali stress serves as an important factor that limits the growth of plants, and the yield and quality of crops (as it inhibits plant photosynthesis and photorespiration).Citation38 This results in metabolic disorders of ROS ,Citation39 and destroys proteins, nucleic acids, and other structures.Citation40 In the natural environment, soil contains both neutral salts (NaCl and Na2SO4), and alkaline salts (NaHCO3 and Na2CO3) ,Citation41 the ion toxicity of alkaline salts is equivalent to that of neutral salts, but alkaline salts cause more serious harm to plants, mainly due to their higher pH values.Citation42,Citation43 The Songnen Plain in Northeast China is extensively covered by NaHCO3-based saline-alkali soil, where the highly alkaline environment restricts the promotion of mulberry to a great extent.Citation44,Citation45 However, the current research in this field rarely involves the effects of saline-alkali stress on the metabolic process of glutamine/glutamine family in plants. Under this context, it is necessary to reveal the depth mechanism of nitrogen assimilation and the mechanism by which the metabolic process of the glutamine/glutamine family responds to saline-alkali stress; this is of vital significance for regulating the saline-alkali tolerance of plants. Our previous study showed that mulberry was tolerant of neutral salts, but relatively sensitive to alkaline salts based on NaHCO3.Citation38,Citation46–Citation48 To clarify the mechanism of this phenomenon and provide a theoretical basis for the reasonable planting of mulberry, this experiment investigated nitrogen assimilation and the metabolic process of the glutamine/glutamine family of amino acids, combined proteomic technology and plant physiology, explored the effects of NaCl and NaHCO3 stresses at the same Na+ concentration (100 mmol L−1) on nitrogen assimilation, and the metabolic process of the glutamine/glutamine family of amino acids in mulberry leaves. The study mainly focused on the responses of GABA, Pro, PAs, and other metabolic processes to NaCl and NaHCO3 stresses, aiming to identify the mechanism of the effects of NaCl and NaHCO3 stresses on nitrogen metabolismin mulberry leaves, and to reveal the adaptation mechanism of the seedling leaves of mulberry under NaCl and NaHCO3 stresses through nitrogen metabolism.
1 Materials and methods
1.1 Experimental materials and treatments
This experiment was carried out in the Soil Science Laboratory of Northeast Agricultural University (Harbin, China) in 2018. One-year-old mulberry seedlings were selected and planted in plastic pots with a diameter of 30 cm and a height of 28 cm. Two plants were planted in each pot, with a seedling height of about 30 cm. The culture medium was fully mixed with peat soil and perlite at a volume ratio of 1:1. NaCl or NaHCO3 treatment were irrigated with 1 L NaCl or NaHCO3 solutions at a concentration of 100 mM (Na+ content is 2.3 gkg−Citation1). The salt concentration and irrigation volume were simulated according to the types and contents of the main salt in saline-alkali soil of Songnen Plain in China. A plastic tray was attached under each pot to prevent any loss of salt/alkali solution. Any solution found in the tray was poured back into the culture medium. The remaining five pots were irrigated with the same volume of distilled water (1 L for each pot) and used as the control (recorded as CK). On the 7th day after irrigation, the parameters were measured after differences in growth phenotypes were apparent.Citation38
1.2 Parameters and measuring methods
Determination of total nitrogen (TN): plant leaves are dried at 80 °C, crushed and screened at 40 mesh, digested with concentrated H2SO4-H2O2, and determined by Micro-Kjeldahl method.Citation49 Determination of soluble protein (SP) and proline [Pro) content using fresh samples by Citation50. H2O2 content was determined as described in Citation51. Nitrate reductase (NR], nitrite reductase (NiR), glutaminesynthetase (GS), glutamate synthase (GOGAT) activity and reduced glutathione (GSH) content were determined using the kits produced by Suzhou Comin Biotechnology Co., Ltd (Suzhou, Jiangsu, China). Determination of putrescine (Put), spermidine (Spd) and spermine (Spm) content: Add 4 ml of precooled 5% perchloric acid to the leaves of fresh mulberry seedlings, grind in ice bath and centrifugate at 4 °C for 30 min (15000 × g). Take 500 µl supernatant and add it into 10 ml centrifuge tube, then add 7 µl benzoyl chloride and 1 ml 2 M NaOH, swirl for 20 s, then react in water bath at 37 °C for 20 min.Add 2 ml of saturated NaC1 solution and mix well, add 2 ml of ether for extraction, centrifugate 1500 × g for 5 min, take 1 ml of ether phase for vacuum drying, dissolve with 100 μl methanol, and then pass through 0.45 μM filter membrane. The content of putrescine (Put), spermidine (Spd) and spermine (Spm) were determined by high-performance liquid chromatography (HPLC, Agilent),in which the chromatographic column was Spherisorb C18 (Waters 3.9 × 150 mm, 10 µm),the UV detection wavelength was 230 nm, the mobile phase was methanol: water = 60:40 (V/V), the flow rate was 0.7 ml min−1, and the injection volume was 20 μl. All the above indexes were measured three times of biological repetition.
Determination and analysis of proteomic:The leaves of mulberry seedlings in different treatments were collected and pre-cooled with liquid nitrogen, and then sent to PTM Biolabs in Hangzhou Eco & Tech Developmental Area [Hangzhou, Zhejiang, China) in an incubator with dry ice for proteomics determination. Proteins related to nitrogen assimilation and glutamine/glutamine family of amino acids metabolism that showed differential expression by 1.2-fold were subjected to further analysis according to Citation38.
qRT-PCR analysis: Based on the findings of proteomics analysis, we chose nitrogen assimilation and glutamine/glutamine family of amino acids metabolism proteins for RT-PCR analysis for the verification of proteomics data according to Citation46. Gene primers sequences (5ʹ-3ʹ] are as follows:
NRT1.3: F: TCGTCTTCACTGAATGTATCGTCA; R: TACGCCCAAGTGAAAGCTCA.
Fd-NiR: F: TGATCCCAGTTTGCAGAGCC; R: TGCACACTCGAGTTCTTTTTAGGT.
Fd-GOGAT: F: CCGCACGCGTGTGTATATAAAAT; R: GTTTTCATGGGTCTCGCATGT.
GS: F: GCGGATCTGGTTTGGACGTA; R: TGCAGCAGCATGTCTCTTGT.
P5 CS: F: GGAGATTGGCGTTGGGAAGA; R: GGTGACATCGAGCTGGCTAA.
GAD1: F: CATGTGGATGCAGCAAGTGG; R: TTCTCCAAATGACCCACCCG.
OAT: F: CTGGAGAGCACGGAAGTACC; R: GGCTTGGCAAGAACTCCTCT.
SPDS: F: CCGACATATCCAAGTGGTGTCA; R: TCAGAAAGGTTGGCAAGGCA.
1.3 Data processing
Excel and SPSS (22.0) were used to analyze the measured data. All data were the mean ± standard error (SE) of three repetitions, and the differences among different treatments were compared by one-way ANOVA and LSD. Taking P < .05 as significant according to Duncan’s multiple range test, P < .01 as very significant according to Duncan’s multiple range test.
2 Results
2.1 Nitrogen assimilation
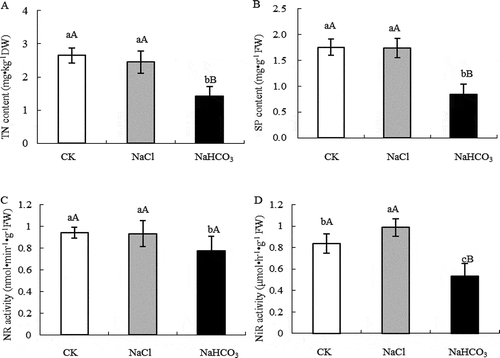
In , TN and SP contents and NR activity of mulberry leaves under NaCl stress did not change significantly, but NiR activity increased by 17.79% (P < .05) compared with CK, while TN, SP content and NR, NiR activity under NaHCO3 stressdecreased very significantly under NaHCO3 stress compared with CK.
Figure 1. TN content (a), SP content (b), NR activity (c) and NiR activity (d) of mulberry leaves under NaCl and NaHCO3 stress. Note: The data are from three replicated experiments (n = 3), and represent means ± SE. Significant differences were expressed by different small letters (P < .05), and very significant differences were expressed by different capital letters (P < .01).
2.2 Glu-Gln cycle
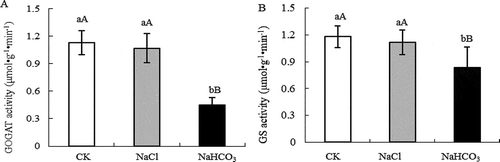
In , the activity of GOGAT and GS in mulberry leaves under NaCl stress did not change significantly, but the activity of GOGAT and GS under NaHCO3 stress decreased by 60.18% (P < .01) and 29.42% (P < .01) respectively compared with CK.
Figure 2. GOGAT activity (a) and GS activity (b) of mulberry leaves under NaCl and NaHCO3 stress. Note: The data are from three replicated experiments (n = 3), and represent means ± SE. Significant differences were expressed by different small letters (P < .05), and very significant differences were expressed by different capital letters (P < .01).
2.3 Pro and PAs metabolism and other metabolic processes of Glu
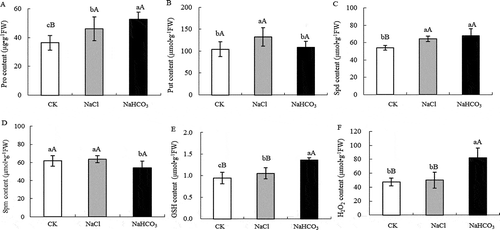
Under NaCl and NaHCO3 stress, the Pro content in mulberry leaves increased by 28.57% (P < .01) and 43.13% (P < .01) respectively compared with CK ().The content of Put and Spd under NaCl stress increased by 26.72% (P < .01) and 18.85% (P < .01) compared with CK, respectively, and the content of Spm did not change significantly. The content of Put and Spd increased slightly compared with CK under NaHCO3 stress, the difference was not significant, but the content of Spm decreased by 11.99% (P < .05) compared with CK (). Under NaCl stress, GSH content increased by 11.66% (P < .05) compared with CK, and H2O2 content did not change significantly. However, under NaHCO3 stress, GSH and H2O2 content were significantly higher than CK and NaCl treatment ().
Figure 3. Pro content (a), Put content (b), Spd content (c), Spm content (d),GSH content (e) and H2O2 content (f) of mulberry leaves under NaCl and NaHCO3 stress. Note: The data are from three replicated experiments (n = 3), and represent means ± SE. Significant differences were expressed by different small letters (P < .05), and very significant differences were expressed by different capital letters (P < .01).
2.4 Nitrogen assimilation and glutamine/glutamine family of amino acids metabolism related enzyme expression
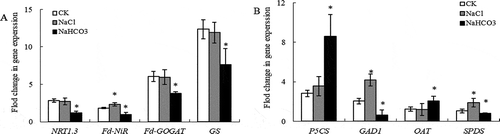
In , under NaCl stress, NRT1.3 expression in mulberry leaves decreased by 25.59% (P < .01), and NRT1.3 expression under NaHCO3 stress further decreased compared with CK and NaCl stress. Under NaCl stress, the expression of NiR and Fd- NiR increased by 27.97% (P < .01) and 21.92% (P < .01) respectively. There was no significant difference between NiR expression compared with CK under NaHCO3 stress, but the expression of Fd-NiR was significantly down-regulated. The expression of Fd-GOGAT, Fd-GOGAT2 and GS (W9SA98 and W9SNP7) under NaCl stress did not change significantly. Under NaHCO3 stress, Fd-GOGAT, Fd-GOGAT2 expression decreased by 47.35% (P < .01) and 47.19% (P < .01), but GS (W9SA98 and W9SNP7) were significantly higher than CK. Under NaCl stress, the expression of P5 CR was higher than CK, there was no significant change in P5 CS expression. The expression of P5 CR and P5 CS increased significantly compared with CK under NaHCO3 stress. During the synthesis of Orn, the expression of ACOAT increased by 18.78% (P < .01) under NaCl stress, but decreased by 18.10% (P < .01) under NaHCO3 stress compared with CK. Under NaCl stress, the change of OAT (W9RH73 and W9QV49) slightly different from those of CK, while under NaHCO3 stress, OAT (W9RH73 and W9QV49) increased significantly. The OTC expression were significantly lower than that of CK under NaCl and NaHCO3 stress. Under NaCl stress, the expression of SPDS did not change significantly, but under NaHCO3 stress, it increased by 35.59% (P < .01) compared with CK. Under NaCl and NaHCO3 stress, the expression of GAD and GAD1 in the leaves of mulberry showed similar trend, which were significantly lower than CK, but the decrease range under NaCl stress was larger than that under NaHCO3 stress. The expression of GCLM increased significantly compared with CK under NaCl and NaHCO3 stress, and the increase of GCLM was more significant under NaHCO3 stress. The expression of GGAT under NaCl stress had no significant change compared with CK, but the expression of GGAT decreased by 21.26% (P < .01) under NaHCO3 stress.
Table 1. Nitrogen assimilation and glutamine/glutamine family of amino acids metabolism related enzymes expression of mulberry leaves under NaCl and NaHCO3 stress.
2.5 qRT-PCR analysis of the key genes expression
NRT1.3, Fd-GOGAT and GS genes expression did not change under NaCl stress compared with CK, NaCl stress increased Fd-NiR gene expression by 19.61% (P < .05) compared with CK, but they were significantly lower than CK under NaHCO3 stress (). Fd-NiR, Fd-GOGAT and GS genes expression were consistent with that of proteins expression under NaCl and NaHCO3stress (). There were no significant difference P5CS and OAT genes expression compared with CK under NaCl stress, GAD1 and SPDS gene expression increased by 103.90% (P < .01) and 79.05% (P < .05), respectively. P5CS and OAT genes expression increased significantly under NaHCO3 stress, but the genes expression of GAD1 and SPDS were significantly lower than CK (). Except for the SPDS gene expression under NaHCO3 stress, the change trend of P5CS, GAD1 and OAT genes expression and protein expression was similar ().
3. Discuss
NR is a key enzyme in the nitrogen assimilation process of plants. It is capable of catalyzing the reduction of NO3− to eventually generate NH4+ and nitrogenous compounds, oxidizing NADH to generate NAD+ for electron transport, and supplementing NAD+ in glycolysis to provide energy for plant growth.Citation52,Citation53 In this experiment, under NaCl stress, NRT1.3 expression in mulberry leaves dropped significantly relative to that in the control leaves (CK), but NR activity did not experience any significant change. In contrast, under NaHCO3 stress, NRT1.3, NRT1.3 gene expression and NR activity both saw a substantial reduction. According to existing studies, NR is an induced enzyme, NO3− is the main signal inducing NR activity, and NR activity declines with the decrease of NO3− in nutrient media.Citation54 By means of lowering the transpiration rate of plants, saline-alkali stress can lower the uptake ratio of NO3–N, and consequently reduce the flow of NO3–N to leaves.Citation55 In this experiment, the decline of NR activity in mulberry leaves under NaHCO3 stress was potentially caused by the inhibition of nitrogen uptake and transport by NaHCO3 in the seedling roots of mulberry (TN and SP contents both dropped significantly). In addition, under NaCl stress, NiR and Fd-NiR and Fd-NiR gene expression in mulberry leaves both increased significantly relative to those in CK, accompanied by a significant increase in NiR activity. However, under NaHCO3 stress, the above nitrogen assimilation process was uniformly inhibited to a very large extent. This partially explains one of our findings in preliminary research, that is, NaHCO3 stress inhibits the growth and photosynthesis of mulberry.Citation38
The Glu-Gln cycle of plants mainly relies on the NH4+ generated by GS and GOGAT through assimilation and photorespiration, and the NH4+ generated by NO3− through reduction in leaves. GS has a very strong affinity for NH4+, which keeps the concentration of NH4+ in plant tissues at an extremely low level.Citation56 In this experiment, NaCl stress did not impose any substantial effect on either GS activity or the expression of GS-associated proteins in mulberry leaves, which suggested that NaCl stress had no substantial effect on the assimilation process of NH4+ in the leaves of mulberry. This guaranteed that the NH4+ accumulated in leaves of mulberry would not cause any toxic effect. However, under NaHCO3 stress, GS expression in the leaves of mulberry increased significantly relative to that in CK, but GS activity dropped significantly. Studies have shown that GS activity is induced by NH4+ ,Citation57,Citation58 so this phenomenon is probably caused by the inhibition of nitrogen uptake and assimilation in the leaves of mulberry under NaHCO3 stress. GOGAT has two forms, NADH-GOGAT using NADH as the electron donor and Fd-GOGAT using Fd as the donor. Fd-GOGAT plays a dominant role in plant leaves and accounts for 95% of GOGAT activity. In this experiment, Fd was used as the electron donor for two GOGAT differential proteins. NaCl stress did not exert any significant effect on either GOGAT activity or the expression of associated proteins in mulberry leaves. In contrast, under NaHCO3 stress, Fd-GOGAT, Fd-GOGAT2 and Fd-GOGAT gene expression in mulberry leavesall decreased significantly, and GOGAT activity also saw a substantial reduction. Fd-GOGAT exists in chloroplasts, and its biosynthesis is photoinduced. It is related to photosynthesis and photorespiration, meaning that factors facilitating photosynthesis also contribute to the expression and synthesis of Fd-GOGAT.Citation59 For this reason, the decline of GOGAT activity and expression in mulberry leaves under NaHCO3 stress might have something to do with the inhibition of its photosynthesis.Citation38,Citation46
Pro has a relatively strong affinity for water and can be used as an osmotic protective agent for plants. The increase in Pro content plays an important role in promoting water uptake by plants.Citation60,Citation61 Pro is synthesized by Glu through catalyzation by pyrroline-5-carboxylate synthase (P5CS) and pyrroline-5-carboxylate reductase (P5CR).Citation62–Citation64 Besides, pyrroline-5-carboxylate (P5C), the precursor of Pro can be synthesized by either Glu, or by Orn and Arg. Under the action of ornithine aminotransferase (OAT), Orn engages in a transamination reaction, generates glutamic-γ- semialdehyde (GSA), and forms P5C.Citation33,Citation65Salt stress and other adversities induce an increase in the expression of plant P5CS and P5CR genes, and promote the synthesis of Pro.Citation66 In contrast, in Arabidopsis after knockout of the P5CS1 gene, the synthesis of Pro is inhibited, and salt tolerance declines.Citation67 A similar conclusion was drawn by Citation68,in their study on rice after knockout of the OSP5CS2 gene. In tobacco, with overexpression of the OAT gene, the accumulation of Pro is tripled, and transgenic tobacco can grow normally in 200 mmol L−1 NaCl.Citation10 In this study, under NaCl stress, P5CR expression in mulberry leaves increased significantly relative to that in CK, while OAT expression did not experience any significant change. In contrast, under NaHCO3 stress, a significant increase was observed in P5CR and P5CS expression, as well as OAT and OAT gene expression. The amplitudes of their increases under NaHCO3 stress were significantly higher than those under NaCl stress. The determination results of Pro content also showed that Pro content in seedling leaves of mulberry under NaHCO3 stress was also significantly higher than under NaCl stress. That is to say, the mulberry leaves could adapt to saline-alkali stress through the accumulation of Pro under both NaCl and NaHCO3 stresses. However, under NaCl stress, the P5C directly synthesized by Glu played the dominant part; under NaHCO3 stress, Orn and Arg played extremely important roles through the P5C synthesized by OAT.
GABA is synthesized mainly by Glu through catalyzation by glutamic acid decarboxylase (GAD). This is also an indirect way of synthesizing GABA: First, pyrroline is formed through the oxygenolysis of PAs by amine oxidase; then pyrroline is further disassimilated into GABA.Citation69 When the synthesis of Gln is blocked, the synthesis of proteins is reduced, and the rate of degradation is lowered in plants. Glu is converted into GABA in a larger amount.Citation70 GABA is also regarded as a signaling molecule that can regulate the growth and development of plants (Lancien and Roberts, Citation71). Some studies have revealed that when a plant is exposed to multiple adversities (both biotic and abiotic), GABA content in the plant will increase rapidly.Citation72,Citation73GABA also induces the accumulation of Pro and PAs. Under salt stress, plants promote Pro synthesis and improve salt tolerance through regulating GABA pathways.Citation74 Exogenous GABA inhibits the generation of ethylene synthesis precursor SAMand thusinduce the accumulation of endogenous PAs [Shi et al., Citation75; Citation76]. According to the results of this experiment, the expression of GAD, GAD1 and GAD1 gene in mulberry leaves were up-regulated under NaCl stress. However, NaHCO3 stress did not experience any significant change. In other words, under NaCl stress, the seedling leaves of mulberry may have increased GABA content and improved salt tolerance by boosting the activity of GAD, but the synthesis of GABA did not exert any protective role under NaHCO3 stress.
The increase of PAs content is a protective response of plants to stress, which enhances the resistance of plants to biotic and abiotic stressors.Citation21,Citation77 PAs also have the function of osmotic regulation, which can help crops to deal with many different adversities and maintain normal physiological activities. Common PAs in plants include Put, Spd, and Spm, etc..Citation78,Citation79 Put has two synthesis pathways in plants: In the first case, it is directly synthesized by Orn through ornithine carbamoyltransferase (ODC); in the second, first putrescine is formed by Arg through catalyzation by arginine carbamoyltransferase (ADC), and then putrescine is hydrolyzed into N-methyl putrescine ammonia (NCP), resulting in the formation of Put. Under adversity stress, the transcription level of the ODC gene in plants is raised,Citation80,Citation81 and overexpression of the ODC gene can improve the salt tolerance of transgenic tobacco.Citation82 Saline-alkali stress also induces increases in ADC expression and PAs content in Brassica juncea.Citation83 Under salt stress, ADC activity and Put contents in rice plants with overexpression of the Avena sativa L. ADC gene were both significantly higher than those in non-GMO plants.Citation84 In this experiment, changes were not directly identified in ODC or ADC expression in mulberry leaves under NaCl and NaHCO3stresses. However, under NaCl stress, the expression of acetylornithine aminotransferase (ACOAT) in mulberry leaves increased. In the process of Arg synthesis by Orn, the expression of key enzyme ornithine carbamoyltransferase (OTC) dropped significantly. Thus, NaCl stress facilitated the synthesis and accumulation of Orn in mulberry leaves, and promoted the direct synthesis of Put. The determination results of Put content also showed that Put content in mulberry leaves under NaCl stress increased significantly relative to that in CK. However, under NaHCO3 stress, Put content did not manifest any significant difference between the seedling leaves of mulberry and CK. This probably could be explained by two aspects: First, the expression of ACOAT declined partially under NaHCO3 stress in the synthesis process of Put; second, the expression of spermidine synthase (SPDS) increased significantly under NaHCO3 stress, causing Put to decompose into Spd. Spd and Spm are formed by Put and decarboxylated S-Adenosylmethionine (SAM) through catalyzation by SPDS and spermine synthase (SPMS).Citation85,Citation86 Plants with overexpression of the SPDS gene usually have an obviously enhanced resistance to multiple abiotic stresses.Citation87,Citation88 In adversities such as saline-alkali stress and drought, SPMS can also raise Spd level, and enhance stress resistance.Citation89,Citation90 In this experiment, the content of Put and Spd in mulberry leaves under NaCl stress increased significantly relative to those in CK. However, under NaHCO3 stress, an increase was observed only in Spd content in mulberry leaves, and Spm content in mulberry leaves dropped significantly relative to that in CK. In other words, under NaCl stress, the seedling leaves of mulberry mostly relied on the Orn pathway for the promotion of Put and Spd synthesis, and the improvement of stress resistance. Under NaHCO3 stress, the leaves adapted to the stress through the accumulation of Spd only.
Saline-alkali stress induces the burst of ROS in plants.Citation91,Citation92 GSH is an important water-soluble antioxidative substance. In plant cells, it can directly reduce some ROS, and eliminate H2O2 through the AsA-GSH cycle.Citation93,Citation94The GSH in plants is synthesized by GCLM through linking up glutamate and glyoxylate.Citation95 This experiment showed that GCLM expression in mulberry leaves increased significantly under both NaCl and NaHCO3stresses. In particular, the amplitude of increase was higher under NaHCO3 stress, and the change in GSH content and GCLM expression presented similar trends. Namely, under NaCl and NaHCO3stressors, the seedling leaves of mulberry could improve their saline-alkali tolerance through promoting GSH synthesis. In this experiment, NaHCO3 stress promoted GSH synthesis in mulberry seedling leaves, and GSH content under NaHCO3 stress was also significantly higher than under NaCl stress. However, H2O2 content in mulberry seedling leaves under NaHCO3 stress was still significantly higher than under NaCl stress, largely due to the increased sources of H2O2 in mulberry seedling leaves under NaHCO3 stress, and also possibly due to the destruction of antioxidative pathways other than GSH. To confirm this, follow-up research will be necessary. The changes of proteins related to nitrogen assimilation and glutamine/glutamine family of amino acids metabolism of mulberry seedlings under NaCl and NaHCO3 stress are shown in .
Figure 5. Schematic presentation of nitrogen assimilation and glutamine/glutamine family of amino acids metabolism of mulberry leaves under NaCl and NaHCO3 stress.
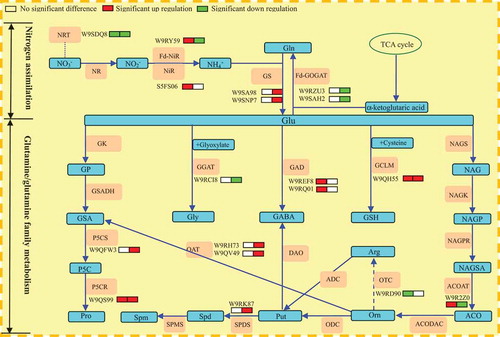
The expression of related proteins are under NaCl and NaHCO3 treatments from left to right. Red represents up-regulated expression,and green indicates for down-regulated expression.
4. Conclusion
NaCl stress did not significant effects on Glu-Gln cycleof mulberry leaves, but the activity and protein expression of NiR were up-regulated. NaCl promotes Pro, Put, GABA and GSH synthesis related enzymes and their gene expression to improve the resistance to NaCl stress. However, under the same Na+ concentration of NaHCO3, the TN and SP contents in mulberry leaves decreased, nitrogen assimilation and Glu-Gln cycle were inhibited, but NaHCO3 stress could induce the increase of Pro and Spd content. Under the stress of NaHCO3, the process of GSH synthesis by Glu was promoted, but the excess H2O2 could not be scavenged effectively. In short, nitrogen assimilation and glutamine/glutamine family of amino acids metabolism in mulberry leaves are sensitive process to NaCl and NaHCO3 stress, the changes of these metabolic processes are important mechanisms for mulberry to adapt to salt and alkali stress.
Abbreviations
| NR | = | nitrate reduce |
| TN | = | total nitrogen |
| SP | = | soluble protein |
| NiR | = | nitrite reduce |
| Fd-NiR | = | ferredoxin-nitrite reductase |
| GS | = | glutamine synthetase |
| GOGAT | = | glutamate synthase |
| Fd-GOGAT | = | ferredoxin-glutamate synthase |
| Pro | = | proline |
| Put | = | putrescine |
| Spd | = | spermidine |
| GAD | = | glutamate decarboxylase |
| GABA | = | γ-aminobutyric acid |
| GCLM | = | glutamate-cysteine ligase |
| GSH | = | glutathione |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Aslam M, Huffaker RC. Dependency of nitrate reduction on soluble carbohydrates in primary leaves of barley under aerobic conditions. Plant Physiol. 1984;75(3):1–11. doi:10.1104/pp.75.3.623.
- Stitt M, Krapp A. The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant Cell Environ. 1999;22:583–621.
- Zilli CG, Balestrasse KB, Yannarelli GG, Polizio AH, Santa-Cruz DM, Tomaro ML. Heme oxygenase up-regulation under salt stress protects nitrogen metabolism in nodules of soybean plants. Environ Exp Bot. 2008;64(1):83–89. doi:10.1016/j.envexpbot.2008.03.005.
- Coleman HD, Cánovas FM, Man HM, Kirby EG, Mansfield SD. Enhanced expression of glutamine synthetase (GS1a) confers altered fibre and wood chemistry in field grown hybrid poplar (Populus tremula×alba) (717-1B4). Plant Biotechnol J. 2012;10(7):883–889. doi:10.1111/j.1467-7652.2012.00714.x.
- Nunes-Nesi A, Fernie AR, Stitt M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol Plant. 2010;3(6):973–996. doi:10.1093/mp/ssq049.
- Xu G, Fan X, Miller AJ. Plant nitrogen assimilation and use efficiency. Annu Rev Plant Biol. 2012;63(1):153–182. doi:10.1146/annurev-arplant-042811-105532.
- Wang H, Zhang M, Guo R, Shi D, Liu B, Lin X, Yang C. Effects of salt stress on ion balance and nitrogen metabolism of old and young leaves in rice (Oryza sativa L.). BMC Plant Biol. 2012;12(1):194. doi:10.1186/1471-2229-12-194.
- Majumdar R, Barchi B, Turlapati SA, Gagne M, Minocha R, Long S, Minocha SC. Glutamate, ornithine, arginine, proline, and polyamine metabolic interactions: the pathway is regulated at the post-transcriptional level. Front Plant Sci. 2016;7:78. doi:10.3389/fpls.2016.00078.
- Rees JD, Ingle RA, Smith JAC. Relative contributions of nine genes in the pathway of histidine biosynthesis to the control of free histidine concentrations in Arabidopsis thaliana. Plant Biotechnol J. 2010;7(6):499–511. doi:10.1111/j.1467-7652.2009.00419.x.
- Hong Z, Lakkineni K, Zhang Z, Verma DP. Removal of feedback inhibition of delta(1)-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol. 2000;122(4):1129–1136. doi:10.1104/pp.122.4.1129.
- Bhaskara GB, Yang TH, Verslues PE. Dynamic proline metabolism: importance and regulation in water limited environments. Front Plant Sci. 2015;6:484. doi:10.3389/fpls.2015.00484.
- Biancucci M, Mattioli R, Moubayidin L, Sabatini S, Costantino P, Trovato M. Proline affects the size of the root meristematic zone in Arabidopsis. BMC Plant Biol. 2015;15(1):263. doi:10.1186/s12870-015-0637-8.
- László S, Arnould S. Proline: a multifunctional amino acid. Trends Plant Sci. 2010;15:89–97.
- Lu Z, Becker DF. Connecting proline metabolism and signaling pathways in plant senescence. Front Plant Sci. 2015;6:522.
- Theocharis A, Clément C, Barka EA. Physiological and molecular changes in plants grown at low temperatures. Planta. 2012;235(6):1091–1105. doi:10.1007/s00425-012-1641-y.
- Kaur G, Asthir B. Proline: a key player in plant abiotic stress tolerance. Biologia Plantarum. 2015;59(4):609–619. doi:10.1007/s10535-015-0549-3.
- Cuin TA, Shabala S. Exogenously supplied compatible solutes rapidly ameliorate NaCl-induced potassium efflux from barley roots. Plant Cell Physiol. 2005;46(12):1924–1933. doi:10.1093/pcp/pci205.
- Nounjan N, Nghia PT, Theerakulpisut P. Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. J Plant Physiol. 2012;169(6):569–604. doi:10.1016/j.jplph.2012.01.004.
- Evelin H, Giri B, Kapoor R. Ultrastructural evidence for AMF mediated salt stress mitigation inTrigonella foenum-graecum. Mycorrhiza. 2013;23(1):71–86. doi:10.1007/s00572-012-0449-8.
- Kishor PBK, Sreenivasulu N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014;37(2):300–311. doi:10.1111/pce.12157.
- Subramanyam S, Sardesai N, Minocha SC, Zheng C, Shukle RH, Williams CE. Hessian fly larval feeding triggers enhanced polyamine levels in susceptible but not resistant wheat. BMC Plant Biol. 2015;15(1):3. doi:10.1186/s12870-014-0396-y.
- Cicatelli A, Lingua G, Todeschini V, Biondi S, Torrigiani P, Castiglione S. Arbuscular mycorrhizal fungi restore normal growth in a white poplar clone grown on heavy metal-contaminated soil, and this is associated with upregulation of foliar metallothionein and polyamine biosynthetic gene expression. Ann Bot. 2010;106(5):791–802. doi:10.1093/aob/mcq170.
- Evelin H, Kapoor R, Giri B. Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann Bot. 2009;104(7):1263–1280. doi:10.1093/aob/mcp251.
- Kusano T, Yamaguchi K, Berberich T. Advances in polyamine research in 2007. J Plant Res. 2007a;120(3):345–350. doi:10.1007/s10265-007-0074-3.
- Minocha R, Majumdar R, Minocha SC. Polyamines and abiotic stress in plants: a complex relationship. Front Plant Sci. 2014;5:175. doi:10.3389/fpls.2014.00175.
- S A R, Tyerman SD, Xu B, Bose J, Kaur S, Conn V, Domingos P, Ullah S, Wege S, Shabala S, et al. GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat Commun. 2015;6:7879.
- Ramesh SA, Kamran M, Sullivan W, Chirkova L, Okamoto M, Degryse F, McLaughlin M, Gilliham M, Tyerman SD. Aluminium-activated malate transporters can facilitate GABA transport. Plant Cell. 2018;30(5):1147–1164. doi:10.1105/tpc.17.00864.
- Gut H, Dominici P, Pilati S, Astegno A, Petoukhov MV, Svergun DI, Grütter MG, Capitani G. A common structural basis for pH-and calmodulin-mediated regulation in plant glutamate decarboxylase. J Mol Biol. 2009;392(2):334–351. doi:10.1016/j.jmb.2009.06.080.
- Shi SQ, Shi Z, Jiang ZP, Qi LW, Sun XM, Li CX, Liu JF, Xiao WF, Zhang SG. Effects of exogenous GABA on gene expression of Caragana intermedia roots under NaCl stress: regulatory roles for H2O2 and ethylene production. Plant Cell Environ. 2010;33(2):149–162. doi:10.1111/j.1365-3040.2009.02065.x.
- Yang A, Cao S, Yang Z, Cai Y, Zheng Y. γ-Aminobutyric acid treatment reduces chilling injury and activates the defence response of peach fruit. Food Chem. 2011;129(4):1619–1622. doi:10.1016/j.foodchem.2011.06.018.
- Jr SMM. Arginine metabolism: boundaries of our knowledge. Journal of Nutr. 2007;137(6):1602–1609. doi:10.1093/jn/137.6.1602S.
- Jr SMM. Recent advances in arginine metabolism: roles and regulation of the arginases. Br J Pharmacol. 2009;157(6):922–930. doi:10.1111/j.1476-5381.2009.00278.x.
- Sakiko O, Dietmar F, Maurizio T,T, Forlani G. Editorial: amino acids of the glutamate family: functions beyond primary metabolism. Front Plant Sci. 2016;7:318.
- Doi K, Kojima T, Makino M, Kimura Y, Fujimoto Y. Studies on the constituents of the leaves of Morus alba L. Chem Pharm Bull (Tokyo). 2001;49(2):151–153. doi:10.1248/cpb.49.151.
- Katsube T, Imawaka N, Kawano Y, Yamazaki Y, Shiwaku K, Yamane Y. Antioxidant flavonol glycosides in mulberry (Morus alba L.) leaves isolated based on LDL antioxidant activity. Food Chem. 2006;97(1):25–31. doi:10.1016/j.foodchem.2005.03.019.
- Li X, Sun ML, Zhang HH, Xu N, Sun GY. Use of Mulberry-soybean intercropping in salt-alkali soil improves the diversity of soil bacterical community. Microbiol Biotechnol. 2016;984:1148–1156.
- Zhang HH, Li X, Zhang SB, Yin ZP, Zhu WX, Li JB, Meng L, Zhong HX, Xu N, Wu YN, et al. Rootstock alleviates salt stress in grafted mulberry seedlings: physiological and PSII function responses. Frontiers Plant Sci. 2018a;9:1806.
- Zhang HH, Shi GL, Shao JY, Xin L, Ma-bo L, Liang M, Nan X, Guang-yu S. Photochemistry and proteomics of mulberry(Morus alba L.) seedlings under NaCl and NaHCO3 stress. Ecotoxicol Environ Saf. 2019;184(30):109624. doi:10.1016/j.ecoenv.2019.109624.
- Zhang HH, Xu N, Li X, Jin WW, Tian Q, Xu N, Sun GY. Overexpression of 2-Cys Prx increased salt tolerance of photosystem II in tobacco. Int J Agri Biol. 2017;19(4):735–745. doi:10.17957/IJAB/15.0348.
- Das SK, Patra JK, Thatoi H. Antioxidative response to abiotic and biotic stresses in mangrove plants: A review. Int Rev Hydrobiol. 2016;101(1–2):3–19. doi:10.1002/iroh.201401744.
- Kim S, Rayburn AL, Voigt T, Parrish A, Lee DK. Salinity Effects on germination and plant growth of prairie cordgrass and switchgrass. BioEnergy Res. 2012;5(1):225–235. doi:10.1007/s12155-011-9145-3.
- Song T, Xu H, Sun N, Jiang L, Tian P, Yong Y, Yang W, Cai H, Cui G. Metabolomic analysis of alfalfa (Medicago sativa L.) root-symbiotic rhizobia responses under alkali stress. Front Plant Sci. 2017;8:1208. doi:10.3389/fpls.2017.01208.
- Zhang HH, Xu N, Wu XY, Wang J, Ma S, Li X, Sun G. Effects of four types of sodium salt stress on plant growth and photosynthetic apparatus in sorghum leaves. J Plant Interact. 2018b;13(1):506–513. doi:10.1080/17429145.2018.1526978.
- Mansour MMF, Ali EF. Evaluation of proline functions in saline conditions. Phytochemistry. 2017;140:52–68. doi:10.1016/j.phytochem.2017.04.016.
- Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59(1):651–681. doi:10.1146/annurev.arplant.59.032607.092911.
- Zhang HH, Wang Y, Li X, Guoqiang H, Yanhui C, Zhiyuan T, Jieyu S, Nan X, Guangyu S. Chlorophyll synthesis and the photoprotective mechanism in leaves of mulberry (Morus alba L.) seedlings under NaCl and NaHCO3 stress revealed by TMT-based proteomics analyses. Ecotoxicol Environ Saf. 2020a;190:110164. doi:10.1016/j.ecoenv.2020.110164.
- Zhang HH, Li X, Wang Y, Mabo L, Yue W, Meijun A, yuehui Z, Guanjun L, Nan X, Guangyu S, et al. Physiological and proteomic responses of reactive oxygen species and antioxidant machinery in leaves of mulberry (Morus alba L.) to NaCl and NaHCO3 stress. Ecotoxicol Environ Saf. 2020b;193:110259. doi:10.1016/j.ecoenv.2020.110259.
- Zhang HH, Li X, Che YH,Wang Y, Li MB, Yang RY, Xu N, Sun GY. A study on the effects of salinity and pH on PSII function in mulberry seedling leaves under saline-alkali mixed stress. Trees-Struct Funct. 2020c;34(3):693–706.
- Bao SD. Soil analysis. Beijing: China agriculture press; 2005.
- Wang JY, Ao H, Zhang J. The echnology and Experiment Principle Of Plant Physiology. Haerbin: Northeast Forestry University press; 2003.
- Alexieva V, Sergiev I, Mapelli S, Karanov E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001;24(12):1337–1344. doi:10.1046/j.1365-3040.2001.00778.x.
- Botrel A, Magne C, Kaiser WM. Nitrate reduction, nitrite reduction and ammonium assimilation in barley roots in response to anoxia. Plant Physiol Biochem. 1996;32:645–652.
- Datta R, Sharma R. Temporal and spatial regulation of nitrate reductase and nitrite reductase in greening maize leaves. Plant Sci. 1999;144(2):77–83. doi:10.1016/S0168-9452(99)00057-6.
- Malagoli M, Canal AD, Quaggiotti S, Pegoraro P, Bottacin A. Differences in nitrate and ammonium uptake between Scots pine and European larch. Plant Soil. 2000;221(1):1–3.
- Fan HF, Guo SR, Du CX, Jiao YS, Li NN, Duan JJ. Effects of exogenous NO on NO3−/NH4+ and soluble protein contents and NR activities in cucumber seedlings under NaCl stress. Acta Botanica Boreali-Occidentalia Sinica. 2006;26(10):2063–2068.
- Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A. Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot. 2010;105(7):1141–1157. doi:10.1093/aob/mcq028.
- Appenroth KJ, Meco R, Jourdan V, Lillo C. Phytochrome and post-translational regulation of nitrate reductase in higher plants. Plant Sci. 2000;159(1):51–56. doi:10.1016/S0168-9452(00)00323-X.
- Ortega JL. Constitutive overexpression of cytosolic glutamine synthetase (GS1) gene in transgenic alfalfa demonstrates that GS1 may be regulated at the level of RNA stability and protein turnover. Plant Physiol. 2001;126(1):109–121. doi:10.1104/pp.126.1.109.
- Sechley KA, Yamaya T, Oaks A. Compartmentation of nitrogen assimilation in higher plants. Int Rev Cytol. 1992;134:85–163.
- Medina A, Roldán A, Azcón R. The effectiveness of arbuscular-mycorrhizal fungi and Aspergillus niger or Phanerochaete chrysosporium treated organic amendments from olive residues upon plant growth in a semi-arid degraded soil. J Environ Manage. 2010;91(12):2547–2553. doi:10.1016/j.jenvman.2010.07.008.
- Miransari M. Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biol. 2010;12(4):563–569. doi:10.1111/j.1438-8677.2009.00308.x.
- Kishor P, Hong Z, Miao GH, Hu C, Verma D. Overexpression of [delta]-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol. 1995;108(4):1387–1394. doi:10.1104/pp.108.4.1387.
- Milosz R, Boguslaw N, Giuseppe F, Zbigniew Z. The structure of Medicago truncatula δ1-pyrroline-5-carboxylate reductase provides new insights into regulation of proline biosynthesis in plants. Front Plant Sci. 2015;6:869.
- Yoshiba Y, Kiyosue T, Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K. Regulation of levels of proline as an osmolyte in plants under water stress. Plant Cell Physiol. 1997;38(10):1095–1102. doi:10.1093/oxfordjournals.pcp.a029093.
- Song SQ, Lei YB, Tian XR. Proline metabolism and cross-tolerance to salinity and heat stress in germinating wheat seeds. Russian J Plant Physiol. 2005;52(6):793–800. doi:10.1007/s11183-005-0117-3.
- Chen THH, Murata N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr Opin Plant Biol. 2002;5(3):250–257. doi:10.1016/S1369-5266(02)00255-8.
- Székely G, Ábrahám E, Rigó G, Zsigmond L, Csiszár J, Ayaydin F, Strizhov N, Jásik S, Schmelzer E, Koncz C, et al. Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J. 2008;53(1):18. doi:10.1111/j.1365-313X.2007.03360.x.
- Junghe HA, Kihong JA, Choonhwan LB, An G. Stress-inducible OsP5CS2 gene is essential for salt and cold tolerance in rice. Plant Sci. 2004;167(3):417–426. doi:10.1016/j.plantsci.2004.04.009.
- Gemperlova L, Eder JM, Cvikrová M. Polyamine metabolism during the growth cycle of tobacco BY-2 cells. Plant Physiol Biochem. 2005;43(4):375–381. doi:10.1016/j.plaphy.2005.02.012.
- Wang YH, Garvin DF, Kochian LV. Rapid induction of regulatory and transporter genes in response to phosphorus, potassium. **Plant Physiol. 2002;130:1361–1370.
- Lancien M, Roberts MR. Regulation of Arabidopsis thaliana 14-3-3 gene expression by γ-aminobutyric acid. Plant, Cell and Environment, Cell & Environment, 2006, 29(7):1430–1436. doi:10.1111/j.1365-3040.2006.01526.x
- Bartyzel I, Pelczar K, Paszkowski A. Functioning of the γ-aminobutyrate pathway in wheat seedlings affected by osmotic stress. Biologia Plantarum. 2003;47(2):221–225. doi:10.1023/B:BIOP.0000022255.01125.99.
- Bown AW, Macgregor KB, Shelp BJ. Gamma-aminobutyrate: defense against invertebrate pests? Trends Plant Sci. 2006;11(9):0–427. doi:10.1016/j.tplants.2006.07.002.
- Lei P, Xu Z, Liang J, Luo X, Zhang Y, Feng X, Xu H. Poly (γ-glutamic acid) enhanced tolerance to salt stress by promoting proline accumulation in Brassica napus L. Plant Growth Regul. 2016;78(2):233–241. doi:10.1007/s10725-015-0088-0.
- Shi SQ, Shi Z, Jiang ZP, et al. Effects of exogenous GABA on gene expression of Caragana intermedia roots under NaCl stress: Regulatory roles for H2O2 and ethylene production. Plant Cell and Environment, 2009, 33(2):149–162.doi: doi:10.1111/j.1365-3040.2009.02065.x
- Turano FJ, Kramer GF, Wang CY. The effect of methionine, ethylene and polyamine catabolic intermediates on polyamine accumulation in detached soybean leaves. Physiol Plant. 2010;101(3):510–518. doi:10.1111/j.1399-3054.1997.tb01031.x.
- Groppa MD, Benavides MP. Polyamines and abiotic stress: recent advances. Amino Acids. 2008;34(1):35–45. doi:10.1007/s00726-007-0501-8.
- Kusano T, Yamaguchi K, Berberich T, Takahashi Y. The polyamine spermine rescues Arabidopsis from salinity and drought stresses. Plant Signal Behav. 2007b;2(4):251–252. doi:10.4161/psb.2.4.3866.
- Larher FR, Aziz A, Gibon Y, Trotel-Aziz P, Bouchereau A. An assessment of the physiological properties of the so-called compatible solutes using in vitro experiments with leaf discs. Plant Physiol Biochem. 2003;41(6–7):657–666.
- Yoda H, Hiroi Y, Sano H. Polyamine Oxidase is one of the key elements for oxidative burst to induce programmed cell death in tobacco cultured cells. Plant Physiol. 2006;142(1):193–206. doi:10.1104/pp.106.080515.
- Yoo TH, Park C-J, Ham B-K, Kim K-J, Paek K-H. Ornithine decarboxylase gene (CaODC1) is specifically induced during TMV-mediated but salicylate-independent resistant response in hot pepper. Plant Cell Physiol. 2004;45(10):1537–1542. doi:10.1093/pcp/pch176.
- Kumria R, Rajam MV. Ornithine decarboxylase transgene in tobacco affects polyamines, in vitro-morphogenesis and response to salt stress. J Plant Physiol. 2002;159(9):983–990. doi:10.1078/0176-1617-00822.
- Mo H, Pua EC. Up-regulation of arginine decarboxylase gene expression and accumulation of polyamines in mustard (Brassica juncea)in response to stress. Physiol Plant. 2002;114(3):439–449. doi:10.1034/j.1399-3054.2002.1140314.x.
- Roy M, Wu R. Arginine decarboxylase transgene expression and analysis of environmental stress tolerance in transgenic rice. Plant Sci. 2001;160(5):869–875. doi:10.1016/S0168-9452(01)00337-5.
- Martin-Tanguy J. Metabolism and function of polyamines in plants: recent development (new approaches). Plant Growth Regul. 2001;34(1):135–148. doi:10.1023/A:1013343106574.
- Shao L, Bhatnagar P, Majumdar R, Minochaet R, Minocha SC. Putrescine overproduction does not affect the catabolism of spermidine and spermine in poplar and Arabidopsis. Amino Acids. 2014;46(3):743–757. doi:10.1007/s00726-013-1581-2.
- Wen X-P, Ban Y, Inoue H, Matsuda N, Moriguchi T. Aluminum tolerance in a spermidine synthase-overexpressing transgenic European pear is correlated with the enhanced level of spermidine via alleviating oxidative status. Environ Exp Bot. 2009;66(3):471–478. doi:10.1016/j.envexpbot.2009.03.014.
- Yang J, Zhang J, Liu K, Wang Z, Liu L. Involvement of polyamines in the drought resistance of rice. J Exp Bot. 2007;58(6):1545–1555. doi:10.1093/jxb/erm032.
- Capell T, Bassie L, Christou P. Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc National Acad Sci. 2004;101(26):9909–9914. doi:10.1073/pnas.0306974101.
- Koji Y, Yoshihiro T, Thomas B, Imai A, Miyazaki A, Takahashi T, Michael A, Kusano T. The polyamine spermine protects against high salt stress in Arabidopsis thaliana. FEBS Lett. 2006;580(30):6783–6788. doi:10.1016/j.febslet.2006.10.078.
- Foyer CH, Noctor G. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant. 2010;119(3):355–364. doi:10.1034/j.1399-3054.2003.00223.x.
- Møller IM. Plant mitochondria and oxidative stress: electron transport, nadph turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol. 2001;52(4):561–591. doi:10.1146/annurev.arplant.52.1.561.
- Sorkheh K, Shiran B, Rouhi V, Khodambashi M, Sofo A. Salt stress induction of some key antioxidant enzymes and metabolites in eight Iranian wild almond species. Acta Physiologiae Plantarum. 2012;34(1):203–213. doi:10.1007/s11738-011-0819-4.
- Zhang HH, Xu ZS, Huo YZ, Guo KW, He GQ, Sun HW, Li MB, Li X, Xu N, Sun GY. Overexpression of Trx CDSP32 gene promotes chlorophyll synthesis and photosynthetic electron transfer and alleviates cadmium-induced photoinhibition of PSII and PSI in tobacco leaves. J Hazard Mater. 2020d;397:122899.
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48(12):909–930. doi:10.1016/j.plaphy.2010.08.016.