ABSTRACT
Casparian strip (CS) is a lignified structure localized on the cell wall between adjacent root endodermal cells and functions as an apoplastic diffusion barrier in the root. The polarly localized, lignin-based CS is an excellent system for studying peptide signaling and position recognition. In this short review, we summarize advances in the past decade on the molecular mechanism governing CS development. In addition to the multi-protein framework underlying the CS membrane domain, we discuss recently observed participation of cell wall located cuproproteins in CS formation. These new discoveries shed light on a potential CS wall domain that coordinates with the membrane domain to provide bidirectional positional information for guiding precise CS development.
Introduction
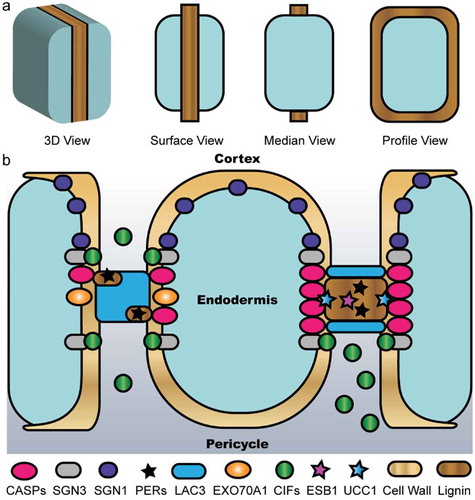
Plants must respond and adapt to various soil conditions for survival because they are sessile and rely on the roots to acquire water and mineral nutrients.Citation1 The protective sheath called Casparian strip (CS) between adjacent endodermal cells allows the root to block the apoplastic pathway and take in minerals selectively.Citation2 This apoplastic diffusion barrier is formed by impregnating the cell wall with lignin, a hydrophobic phenylpropanoid polymer that seals the entire intercellular space between two neighboring cytoplasmic membranes.Citation3 CS occupies the central region of transversal and anticlinal cell walls of endodermal cellsCitation4 (). Collectively, connected CSs are woven into a fishnet-like structure that stretches from the differentiation zone to the root-shoot junction.Citation4 The mechanism underlying the establishment of a polarly localized, lignin-based CS has been an intriguing question since 1865, when it was first described by the German botanist Robert Caspary.Citation5
Figure 1. Schematic representation of the multi-protein framework for CS development. (a) The morphology of CS observed from different views. (b) Model for how the wall domain coordinates with the membrane domain to spatially define CS. Before the diffusion barrier is established, as shown on the left portion, SGN3 and SGN1 recognize the CIF peptides emanating from the stele to act as a surveillance system that activates localized ROS and downstream gene expression. As shown on the right portion, LAC3 participates in forming the cell wall domain, which together with EXO70A1 help focalize CASPs to demark the CS membrane domain and recruit PER64 for further lignification and proper sealing of the extracellular space. ESB1 and UCC1 are involved in this scenario with unknown functions. Once the CS is sealed, the CIF peptides are trapped in the pericycle side and thus cease signaling.
The upstream signaling pathway for CS formation
The first proteins found to be directly involved in CS development are Casparian strip membrane domain proteins (CASPs). Five members of this family in Arabidopsis, CASP1-5, mark a membrane domain that is attached to CS and excludes most other plasma membrane proteins.Citation6,Citation7 The interaction between CASP1 and CASP3 is thought to form a scaffold to recruit other proteins into the CS region. These characteristics of CASPs are conserved in other plant species such as rice and tomato.Citation8–Citation11 To search for genes that regulate CS development, Niko Geldner and colleagues developed an ingenious assay to screen for mutants with leaky CS, employing the stele active promoter proSHR to drive β-glucuronidase as a reporter.Citation12 Four genes were identified from this screen and named SCHENGEN (SGN). SGN4 encodes an NADPH oxidase (also known as RBOHF) and its mutants display severe delay of lignin deposition on CS.Citation13 Pharmacological inhibition of other components of the ROS pathway as well as generation of a quintuple peroxidase (PER) mutant abolishes lignin deposition on CS,Citation13,Citation14 firmly establishing peroxidases as the main enzymes for lignin crosslinking in CS. SGN3 is a leucine-rich-repeat receptor-like kinase flanking the central CS membrane domain,Citation15 whereas SGN1 localizing to the outer plasma membrane belongs to the receptor-like cytoplasmic kinase family.Citation12 The ligands for SGN3, named CASPARIAN STRIP INTEGRITY FACTOR 1 & 2 (CIF1 & 2), are small peptides expressed from the stele.Citation16,Citation17 These peptides are sulfated by SGN2, the only tyrosylprotein sulfotransferase in Arabidopsis.Citation18 Function of the CIF-SGN3-SGN1 signaling module has been elucidated. On one hand, SGN3 bound by CIFs phosphorylates SGN1, which furtherly phosphorylates and activates RBOHF and RBOHD to induce localized ROS production for lignin deposition. On the other hand, CIF/SGN3 induces MAP kinase phosphorylation and activates downstream gene expression.Citation19 Once the CS is sealed, the CIF peptides physically separate from the receptor. Thus, the CIF-SGN3-SGN1 module acts as a surveillance system to detect porous CS and trigger compensatory lignification that starts at the middle lamellae of cell cornersCitation18,Citation20 (). It is subsequently shown that this signaling pathway acts in parallel with the SHORTROOT-SCARECROW (SHR-SCR) module to control endodermis identity altogether.Citation21,Citation22
Other CS-associated proteins
Mutants resulting in discontinuous CS alter the elemental profile in the shoot, which is often coupled with enhanced suberin deposition at the CS, presumably to compensate for the compromised integrity of the lignin ring. This phenomenon in retrospect has inspired reexamination of mutants with altered elemental profiles for isolating determinants of CASP localization and CS formation. Enhanced suberin1 (ESB1) was characterized this way.Citation23 ESB1 is a dirigent domain-containing protein with unknown function and specifically localizes to the CS. MYB36 is a transcription factor induced by the SHR-SCR module and activates the expression of many CS related genes such as CASPs, PER64 and ESB1.Citation24,Citation25 Among another set of mutants (termed lord of the rings) that compromises CS integrity, LOTR1 is a member of an uncharacterized family and LOTR2 is a subunit of the exocyst complex.Citation26,Citation27 LOTR2/EXO70A1 transiently accumulates on CS membrane domain prior to CASP1. In its absence, CASP1 secretion is not affected but displays dramatic de-localization into random microdomains, indicating that EXO70A1 generates a transient positional information that guides CASP localizationCitation27 (). Despite these findings, how the various factors integrate together to form a polarly localized CASP domain remains to be elucidated.
CS localized cuproproteins
Lignin is a polymer formed by the dehydrogenative polymerization of monolignols.Citation28 Many essential phenylpropanoid biosynthesis genes are expressed in the root endodermis, suggesting that this cell layer is able to synthesize monolignols autonomously.Citation29 A monolignol transporter AtABCG29 is found localizing on the plasma membrane of endodermis,Citation30 though monolignols can also be exported via passive membrane crossing.Citation31 Taken together, these observations indicate that precise positioning of CS lignification is not dependent on polar transport of lignin monomers, but localized monolignol oxidation. Laccases are secreted multi-copper oxidases that have been implicated in oxidative coupling of monolignols within the cell wall.Citation28 A particular member of the laccase family in Arabidopsis, LAC3, is specifically expressed in the endodermis and its transcript abundance is further upregulated by CIF/SGN3 signaling,Citation19 suggesting that LAC3 participates in CS development. Recently, it was reported that LAC3 localizes on the primary cell wall in the CS domain, initially preceding CASP1 localization to the membrane, then accumulating between adjacent CASP1 domains, and eventually localizing on the borders of CS.Citation32 Moreover, copper chelation and inhibition of N-glycosylation cause CASP1 dispersal and abnormal lignification.Citation32 These findings indicate that cuproproteins are required for CASP1 focalization and proper CS lignification. However, a nonuple mutant of LAC genes in Arabidopsis exhibits no apparent defects in CS formation,Citation14 implying that additional cuproproteins are involved.
Uclacyanins (UCCs) are a glycosylphosphatidylinositol-anchored sub-family of plant-specific blue copper proteins possessing a characteristic mononuclear copper site and a putative arabinogalactan glycomodule resembling that of cell wall structural proteins.Citation33 Thus, UCCs are likely anchored to the cell surface and associate with the cell wall. Transcriptomic data in Arabidopsis indicate that UCC1 is differentially expressed in myb36 mutants.Citation24,Citation34 UCC1 is compartmentalized and forms a central nanodomain within the CS in contrast to LAC3 and other CS-located proteins that mainly accumulate at the periphery of the CSCitation32,Citation34 (). Loss-of-function of UCC1 & 2 reduces lignification specifically in this central CS nanodomain,Citation34 similar to CS defects caused by copper chelation.Citation32 These observations suggest that strong toxicity of copper chelation on CS development may reflect a comprehensive effect of interfering both LAC3 and UCC1 & 2 or other yet-unidentified cuproproteins. Interestingly, the transcripts of LAC3 as well as its close homologs LAC12 & 13 and UCC1 are targeted by a conserved microRNA, miR408.Citation35 This miRNA belongs to the so-called copper-miRNAs because they mostly target transcripts encoding cuproproteins and their abundance is regulated by the copper-responsive transcription factor SQUAMOSA PROMOTER BINDING PROTEIN-LIKE7.Citation36,Citation37 These findings indicate that regulation and function of LAC3 and UCC1 & 2 are orchestrated.
Different from tight junctions in animal cells that are directly sealed by large protein complexes, two adjacent root endodermal cells share one CS position with the plasma membranes physically separated by the cell wall. Thus, it was proposed that cuproproteins such as LAC3 marks a possible cell wall domain that coordinates with the membrane domain to define the positional information for accurate CS formationCitation32 (). In this proposal, anchoring LAC3 to the cell wall is one of the earliest events in CS development. LAC3 and UCC1 & 2 compartmentalized respectively to the periphery and the central nanodomain of the CS are hypothesized to define a cell wall domain that provides an interface for restricting or priming the peroxidase pathway for CS lignification (). Likewise, a study using scr mutants supports the notion that endodermal cell-cell contact is required for the positioning of CS.Citation38 It can be extrapolated that this cell wall domain coordinates with the CASP domain and contributes to CS integrity that is under surveillance by the CIF-SGN3 peptide signaling pathway.Citation32 Thus, precise CS lignification across a cell wall in between neighboring endodermal cells may require separate wall domain and membrane domain that coordinate to provide bidirectional positional information.
Conclusions
During the last decade, studies of the molecular mechanism underlying CS development have made impressive progress. So far, most of the experimentally identified CS factors show specific localization on the plasma membrane of endodermal cells. Despite the long debate on whether laccases or peroxidases oxidize monolignols to polymerize lignin,Citation14,Citation28 recent characterization of cuproproteins exemplified by LAC3 and UCC1 supports a model for CS positioning involving a cell wall domain (). This notion brings copper homeostasis, which consists of sophisticated gene regulation programs and elaborate transport systems,Citation39,Citation40 into the realm of CS development. Further elucidation of the function of CS cuproproteins and related copper homeostatic events should provide more insights into the cellular mechanisms governing position recognition and focalized lignification in plants.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Petricka JJ, Winter CM, Benfey PN. Control of Arabidopsis root development. Annu Rev Plant Biol. 2012;63:1–4. doi:10.1146/annurev-arplant-042811-105501.
- Doblas VG, Geldner N, Barberon M. The endodermis, a tightly controlled barrier for nutrients. Curr Opin Plant Biol. 2017;39:136–143. doi:10.1016/j.pbi.2017.06.010.
- Naseer S, Lee Y, Lapierre C, Franke R, Nawrath C, Geldner N. Casparian strip diffusion barrier in Arabidopsis is made of a lignin polymer without suberin. Proc Natl Acad Sci USA. 2012;109:10101–10106. doi:10.1073/pnas.1205726109.
- Geldner N. The endodermis. Annu Rev Plant Biol. 2013;64:531–558. doi:10.1146/annurev-arplant-050312-120050.
- Caspary MR. Bemerkungen über die Schutzscheide und die Bildung des Stammes und der Wurzel. Jahrbücher für wissenschaftliche Botanik. 1865;4:101–124.
- Roppolo D, De Rybel B, Denervaud Tendon V, Pfister A, Alassimone J, Vermeer JE, Yamazaki M, Stierhof Y-D, Beeckman T, Geldner N, et al. A novel protein family mediates Casparian strip formation in the endodermis. Nature. 2011;473:380–383. doi:10.1038/nature10070.
- Alassimone J, Naseer S, Geldner N. A developmental framework for endodermal differentiation and polarity. Proc Natl Acad Sci USA. 2010;107:5214–5219. doi:10.1073/pnas.0910772107.
- Roppolo D, Boeckmann B, Pfister A, Boutet E, Rubio MC, Dénervaud-Tendon V, Vermeer JEM, Gheyselinck J, Xenarios I, Geldner N, et al. Functional and evolutionary analysis of the CASPARIAN STRIP MEMBRANE DOMAIN PROTEIN family. Plant Physiol. 2014;165:1709–1722. doi:10.1104/pp.114.239137.
- Wang Z, Yamaji N, Huang S, Zhang X, Shi M, Fu S, Yang G, Ma JF, Xia J. OsCASP1 is required for casparian strip formation at endodermal cells of rice roots for selective uptake of mineral elements. Plant Cell. 2019;31:2636–2648. doi:10.1105/tpc.19.00296.
- Wang Z, Shi M, Wei Q, Chen Z, Huang J, Xia J. OsCASP1 forms complexes with itself and OsCASP2 in rice. Plant Signal Behav. 2020;15:1706025. doi:10.1080/15592324.2019.1706025.
- Li P, Yang M, Chang J, Wu J, Zhong F, Rahman A, Qin H, Wu S. Spatial expression and functional analysis of casparian strip regulatory genes in endodermis reveals the conserved mechanism in tomato. Front Plant Sci. 2018;9. doi:10.3389/fpls.2018.00832.
- Alassimone J, Fujita S, Doblas VG, van Dop M, Barberon M, Kalmbach L, Vermeer JEM, Rojas-Murcia N, Santuari L, Hardtke CS, et al. Polarly localized kinase SGN1 is required for Casparian strip integrity and positioning. Nat Plants. 2016;2:16113. doi:10.1038/nplants.2016.113.
- Lee Y, Rubio MC, Alassimone J, Geldner N. A mechanism for localized lignin deposition in the endodermis. Cell. 2013;153:402–412. doi:10.1016/j.cell.2013.02.045.
- Rojas-Murcia N, Hématy K, Lee Y, Emonet A, Ursache R, Fujita S, De Bellis D, Geldner N. High-order mutants reveal an essential requirement for peroxidases but not laccases in Casparian strip lignification. bioRxiv. 2020;2020.06.17.154617. doi:10.1101/2020.06.17.154617.
- Pfister A, Barberon M, Alassimone J, Kalmbach L, Lee Y, Vermeer JE, Yamazaki M, Li G, Maurel C, Takano J, et al. A receptor-like kinase mutant with absent endodermal diffusion barrier displays selective nutrient homeostasis defects. Elife. 2014;3:e03115. doi:10.7554/eLife.03115.
- Nakayama T, Shinohara H, Tanaka M, Baba K, Ogawa-Ohnishi M, Matsubayashi Y. A peptide hormone required for Casparian strip diffusion barrier formation in Arabidopsis roots. Science. 2017;355:284–286. doi:10.1126/science.aai9057.
- Okuda S, Fujita S, Moretti A, Hohmann U, Doblas VG, Ma Y, Pfister A, Brandt B, Geldner N, Hothorn M, et al. Molecular mechanism for the recognition of sequence-divergent CIF peptides by the plant receptor kinases GSO1/SGN3 and GSO2. Proc Natl Acad Sci USA. 2020;117:2693–2703. doi:10.1073/pnas.1911553117.
- Doblas VG, Smakowska-Luzan E, Fujita S, Alassimone J, Barberon M, Madalinski M, Belkhadir Y, Geldner N. Root diffusion barrier control by a vasculature-derived peptide binding to the SGN3 receptor. Science. 2017;355:280–284. doi:10.1126/science.aaj1562.
- Fujita S, De Bellis D, Edel KH, Köster P, Andersen TG, Schmid-Siegert E, Dénervaud Tendon V, Pfister A, Marhavý P, Ursache R, et al. SCHENGEN receptor module drives localized ROS production and lignification in plant roots. Embo J. 2020;39:e103894. doi:10.15252/embj.2019103894.
- Wang P, Calvo-Polanco M, Reyt G, Barberon M, Champeyroux C, Santoni V, Maurel C, Franke RB, Ljung K, Novak O, et al. Surveillance of cell wall diffusion barrier integrity modulates water and solute transport in plants. Sci Rep. 2019;9:4227. doi:10.1038/s41598-019-40588-5.
- Drapek C, Sparks EE, Marhavy P, Taylor I, Andersen TG, Hennacy JH, Geldner N, Benfey PN. Minimum requirements for changing and maintaining endodermis cell identity in the Arabidopsis root. Nat Plants. 2018;4:586–595. doi:10.1038/s41477-018-0213-y.
- Li P, Yu Q, Gu X, Xu C, Qi S, Wang H, Zhong F, Baskin TI, Rahman A, Wu S, et al. Construction of a functional casparian strip in non-endodermal lineages is orchestrated by two parallel signaling systems in Arabidopsis thaliana. Curr Biol. 2018;28:2777–86.e2. doi:10.1016/j.cub.2018.07.028.
- Hosmani PS, Kamiya T, Danku J, Naseer S, Geldner N, Guerinot ML, Salt DE. Dirigent domain-containing protein is part of the machinery required for formation of the lignin-based Casparian strip in the root. Proc Natl Acad Sci USA. 2013;110:14498–14503. doi:10.1073/pnas.1308412110.
- Kamiya T, Borghi M, Wang P, Danku JM, Kalmbach L, Hosmani PS, Naseer S, Fujiwara T, Geldner N, Salt DE, et al. The MYB36 transcription factor orchestrates Casparian strip formation. Proc Natl Acad Sci USA. 2015;112:10533–10538. doi:10.1073/pnas.1507691112.
- Liberman LM, Sparks EE, Moreno-Risueno MA, Petricka JJ, Benfey PN. MYB36 regulates the transition from proliferation to differentiation in the Arabidopsis root. Proc Natl Acad Sci USA. 2015;112:12099–12104. doi:10.1073/pnas.1515576112.
- Li B, Kamiya T, Kalmbach L, Yamagami M, Yamaguchi K, Shigenobu S, Sawa S, Danku JMC, Salt DE, Geldner N, et al. Role of LOTR1 in nutrient transport through organization of spatial distribution of root endodermal barriers. Curr Biol. 2017;27:758–765. doi:10.1016/j.cub.2017.01.030.
- Kalmbach L, Hematy K, De Bellis D, Barberon M, Fujita S, Ursache R, Daraspe J, Geldner N. Transient cell-specific EXO70A1 activity in the CASP domain and Casparian strip localization. Nat Plants. 2017;3:17058. doi:10.1038/nplants.2017.58.
- Boerjan W, Ralph J, Baucher M. Lignin biosynthesis. Annu Rev Plant Biol. 2003;54:519–546. doi:10.1146/annurev.arplant.54.031902.134938.
- Andersen TG, Molina D, Kilian J, Franke R, Ragni L, Geldner N. Tissue-autonomous phenylpropanoid production is essential for establishment of root barriers. bioRxiv. 2020:2020.06.18.159475. doi:10.1101/2020.06.18.159475.
- Alejandro S, Lee Y, Tohge T, Sudre D, Osorio S, Park J, Bovet L, Lee Y, Geldner N, Fernie A, et al. AtABCG29 is a monolignol transporter involved in lignin biosynthesis. Curr Biol. 2012;22:1207–1212. doi:10.1016/j.cub.2012.04.064.
- Vermaas JV, Dixon RA, Chen F, Mansfield SD, Boerjan W, Ralph J, Crowley MF, Beckham GT. Passive membrane transport of lignin-related compounds. Proc Natl Acad Sci USA. 2019;116:23117–23123. doi:10.1073/pnas.1904643116.
- Zhuang Y, Zuo D, Tao Y, Cai H, Li L. Laccase3-based extracellular domain provides possible positional information for directing Casparian strip formation in Arabidopsis. Proc Natl Acad Sci USA. 2020:202005429. doi:10.1073/pnas.2005429117.
- Nersissian AM, Valentine JS, Immoos C, Hill MG, Hart PJ, Williams G, Herrmann RG. Uclacyanins, stellacyanins, and plantacyanins are distinct subfamilies of phytocyanins: plant-specific mononuclear blue copper proteins. Protein Sci. 1998;7:1915–1929. doi:10.1002/pro.5560070907.
- Reyt G, Chao Z, Flis P, Castrillo G, Chao D-Y, Salt DE. Uclacyanin proteins are required for lignified nanodomain formation within Casparian strips. bioRxiv. 2020:2020.05.01.071738. doi:10.1101/2020.05.01.071738.
- Pan J, Huang D, Guo Z, Kuang Z, Zhang H, Xie X, Ma Z, Gao S, Lerdau MT, Chu C, et al. Overexpression of microRNA408 enhances photosynthesis, growth, and seed yield in diverse plants. J Integr Plant Biol. 2018;60:323–340. doi:10.1111/jipb.12634.
- Pilon M. The copper microRNAs. New Phytol. 2017;213:1030–1035. Yamasaki H, Hayashi M, Fukazawa M, Kobayashi Y, Shikanai T. SQUAMOSA Promoter Binding Protein-Like7 Is a Central Regulator for Copper Homeostasis in Arabidopsis. Plant Cell 2009; 21:347–361. doi:10.1105/tpc.108.060137. doi:10.1111/nph.14244.
- Zhang H, Zhao X, Li J, Cai H, Deng XW, Li L. MicroRNA408 is critical for the HY5-SPL7 gene network that mediates the coordinated response to light and copper. Plant Cell. 2014;26:4933–4953. doi:10.1105/tpc.114.127340.
- Martinka M, Dolan L, Pernas M, Abe J, Lux A. Endodermal cell-cell contact is required for the spatial control of Casparian band development in Arabidopsis thaliana. Ann Bot. 2012;110:361–371. doi:10.1093/aob/mcs110.
- Burkhead JL, Reynolds KA, Abdel-Ghany SE, Cohu CM, Pilon M. Copper homeostasis. New Phytol. 2009;182:799–816. doi:10.1111/j.1469-8137.2009.02846.x.
- Robinson NJ, Winge DR. Copper metallochaperones. Annu Rev Biochem. 2010;79:537–562. doi:10.1146/annurev-biochem-030409-143539.
