ABSTRACT
Functional genomics can be applied to shed light on the Brassica napus – Verticillium interaction. RNAseq data indicated already that abscisic acid (ABA) is apparently involved in the early oilseed rape response to fungal infection with Verticillium longisporum isolate 43 (Vl43). A calreticulin (CRT1a) was identified as novel susceptibility factor for Vl43 infecting both Arabidopsis and oilseed rape. CRT1a is involved in calcium homeostasis and contributes in the endoplasmatic reticulum to the unfolded protein response. The latter function could either affect the correct folding of other susceptibility factors or of negative regulators in ethylene (ET) signaling. Which CRT1a function is affected in the mutants is currently unknown, but both hypotheses can explain that crt1a loss-of-function mutants display increased resistance to V. longisporum and enhanced expression of ethylene signaling related genes. This indicates that besides other phytohormones such as ABA or salicylic acid (SA) also ET plays a critical role in the plant-Verticillium interaction, which might be exploited to improve plant resistance.
A functional genomics approach to identify novel susceptibility factors involved in host-pathogen interactions
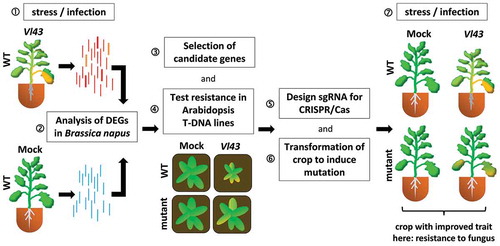
Oilseed rape is a rather young crop species with a complex allotetraploid genome, but low genetic variation which makes resistance breeding very challenging.Citation1 The hemibiotrophic vascular fungal pathogen Verticillium longisporum causes stem striping on oilseed rape and since there is no chemical control, resistance breeding is the method of choice.Citation2 However, the identification of new target genes for genome editing in pathogen resistance breeding is a major bottleneck. Functional genomics can be deployed to overcome this obstacle and identify candidate genes encoding novel susceptibility factors by differential expression analysis (). Plant transcripts might be up-regulated during infection either due to their function in the host defense reaction or due to manipulation by the pathogen to gain an advantage (①). Bioinformatic analysis e.g. of RNAseq experiments, including meta-studies (②), helps to identify up-regulated genes, which are potentially interesting candidate genes for further analysis (③). Since oilseed rape and the model plant Arabidopsis are related Brassicaceous species, a pre-screen deploying Arabidopsis T-DNA mutants can be used to identify promising candidates for genome editing in oilseed rape (④). Mutation of the candidate genes in oilseed rape can be easiest accomplished by genome editing, e.g. via CRISPR/Cas. To achieve this, another bioinformatics approach has to be conducted to select a suitable region in the candidate gene to be targeted by a locus-specific sgRNA (⑤). After crop transformation and regeneration of plants (⑥) it is advisable to propagate the seeds first in order to obtain T2 plants with a more homogenous background concerning the target site mutations. In a final test these plants are challenged with the pathogen or other stress of interest and in best case some plants show resistance to fungal infection depending on the type of mutation (⑦). From 20 selected candidate genes analyzed by deploying corresponding Arabidopsis T-DNA lines in infection assays with the phytopathogenic fungus Verticillium longisporum (Vl43), CRT1a was identified as a novel susceptibility factor for this pathogen.Citation3
Phytohormone crosstalk in the host-Verticillium interaction
To get a clue about the possible mechanism(s) which might be influenced by the CRT1a mutation, expression of phytohormone related marker genes can be investigated and both Brassica napus and Arabidopsis mutants displayed enhanced expression of ethylene (ET) signaling related genes (such as EIN2) as well as higher resistance to Vl43. Comparing the stress-related phytohormone biosynthetic processes in both plant species by RNAseq from wild type plant roots harvested at 6 days post inoculation (dpi) with Vl43, it was found that the hormone responses appeared to differ between the crop and the model plant with respect to abscisic acid (ABA). Upon infection, ABA responses were suppressed in Brassica napus, but not altered in Arabidopsis. Expression of the JA/ET marker gene PDF1.2 in oilseed rape was much less pronounced (4-fold) than in Arabidopsis (200-fold), where this response was similarly strong as the SA response,Citation4 probably because ABA is known to positively influence JA biosynthesis and signaling.Citation5 However, on RNAseq level JA responses did not appear to be differentially regulated comparing both plant species. Expression of the SA marker gene PR1 was up-regulated in both oilseed rape (500-fold) and Arabidopsis (200-fold).Citation4 This could be further explained by mutually antagonistic effects between SA and ABACitation6-Citation10 with SA levels being elevated when ABA is suppressed as observed in oilseed rape during early infection with Vl43 and confirmed by the finding that in the Arabidopsis ABA mutant nced3, PR1 expression was enhanced, while PDF1.2 and EIN2 expression were suppressed compared to Col-0 wild type.Citation4 This was surprising, because also the interaction between ABA and ET signaling is reported to be mutually antagonisticCitation11,Citation12 indicating a dominant negative effect of elevated SA signaling on ET and JA. In Arabidopsis, ABA responses were not suppressed, which is in line with observations that ABA levels tend to increase in Vl43 infected roots at 6 and 8 dpiCitation13 and also in leaves early at 2 dpiCitation14 or later at 15 dpi.Citation15 Generally, ET and JA are believed to act in concert to orchestrate the plant defense response to necrotrophic pathogens and are antagonistically regulated by SA, being responsible for the defense responses to biotrophic pathogens.Citation16,Citation17 Especially the SA inducible WRKY70 has been implicated in negative regulation of JA responses in Arabidopsis,Citation18 wheatCitation19 and cotton.Citation20 Compared to PDF1.2 and PR1 marker gene induction only a weak ET marker gene (EIN2) expression was observed in both Arabidopsis and oilseed rape,Citation4 but over-expression of some ET responsive transcription factors (ERFs) increased Arabidopsis resistance to Vl43Citation21 and other pathogens.Citation22 In crt1a mutants of both plant species enhanced ET marker gene expression goes along with reduced susceptibility, indicating ET plays a more important role than previously anticipated and that there might be extensive phytohormone crosstalk determining the outcome of this compatible interaction. In Arabidopsis it was shown that infection with the Verticillium longisporum isolate VD11 gradually induced ET production and further reduced the fresh weight in the ET mutants ein2-1 (pos. regulator in ET signaling) and ein4-1 (an ET receptor, involved in negative regulation of ET signaling) as compared to Col-0 plants, while fresh weight increased in etr1-1 (another ET receptor, also negatively regulating ET signaling) mutants.Citation23 Though data concerning ET receptor mutants are somewhat ambiguous, all in all it appears that ET is required for the defense response to V. longisporum. In the Arabidopsis – V. longisporum interaction it has been demonstrated by mutant analysis that the fungus requires a COI1-dependent but JA-Ile-independent mechanism to efficiently colonize Arabidopsis.Citation15 JA and MYC2 are required for full induction of monoterpenes production, which can stimulate germination and enhance V. longisporum invasion thus myc2-1 mutants display higher resistance.Citation24 In contrast, the coi1-16 mutant was more susceptible in the screen of various Arabidopsis genotypes and the eds8-1 or jar1-1 mutants were more resistant than Col-0.Citation23 However, the Arabidopsis npr1-1 mutant was more susceptible and showed decreased responses to the ethylene precursor ACC or MeJA pretreatments, indicating a major involvement of the SA pathway in this interaction.Citation23 Furthermore, these authors showed that SA-dependent PR1 was elevated highest at 7 dpi when compared to PDF1.2 and the ET-dependent PR4 expression.Citation23 It is important to note that the outcome of an interaction between phytohormones depends largely on their equilibrium, which could explain sometimes contradicting observations and makes prediction of phytohormone crosstalk difficult. For example, the relationship between ET and SA could be interpreted as being mutually antagonistic since a negative effect of SA on ET has been described in rice,Citation25 pear,Citation26 mustard,Citation27 melon,Citation28 oilseed rapeCitation29 and ArabidopsisCitation30 or negative effects of ET on SA in ArabidopsisCitation31,Citation32 and rice.Citation33 However, other reports indicate positive effects between both hormones, e.g. in ArabidopsisCitation34 and carrotCitation35 (SA on ET) or tomatoCitation36 and ArabidopsisCitation34 (ET on SA), often depending on the concentration of applied phytohormones. Also during pattern triggered immunity (PTI) it has been shown in tobacco that low amounts of PAL-derived salicylic acid are required to induce ET synthesis, while high SA amounts could inhibit the elicitor-induced ET production.Citation37
Conclusion
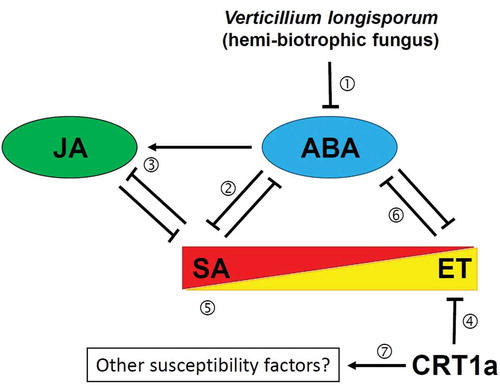
The SA marker gene expression is higher in Brassica napus compared to Arabidopsis,Citation4 which could be explained by reduced ABA levelsCitation10 and since transgenic Brassica napus NahG (SA-deficient) plants displayed increased susceptibility to V. longisporum,Citation38 basal resistance of oilseed rape to V. longisporum could be mainly attributed to this phytohormone. As discussed above, data concerning the JA response with respect to Arabidopsis infection with V. longisporum are not consistent. At least in Brassica napus the PDF1.2 expression was much lower than in Arabidopsis,Citation4 which could be also explained by reduced ABA levels.Citation5 Thus, all these differences between oilseed rape and Arabidopsis may be caused by the suppression of the ABA response which was observed in the oilseed rape – Verticillium interaction. Since ET responses appear to be activated in the crt1a mutants of Arabidopsis and Brassica napus, it is possible that ET presence at the time of infection inhibits disease development, while at later stages it might contribute to wilt development as discussed for tomato and V. dahliae.Citation39 A working model explaining the interplay of ABA, SA, JA and ET in the oilseed rape – Verticillium interaction is presented in .
Figure 2. Working model of phytohormone crosstalk during the Brassica napus – V. longisporum interaction.① ABA is repressed in oilseed rape, but not in Arabidopsis. ② Therefore SA can accumulate higher compared to Arabidopsis. ③ Higher SA and lower ABA levels negatively influence JA, which is stronger activated in Arabidopsis. ④ ET responses could be activated in the crt1a mutants due to improper folding of negative regulators. ⑤ ET might directly benefit SA accumulation or indirectly via negatively affecting ABA ⑥. It has been proposed that the reduced ABA response might help the fungus to establish a long-lasting compatible interaction.Citation4 while the mechanism underlying resistance in the crt1a background is currently unknown and could also involve correct folding of other – yet unknown – susceptibility factors ⑦.Citation3
While manipulating ABA signaling could severely affect plant responses to abiotic stress, the crt1a mutant with enhanced ET signaling did not appear to negatively affect plant physiology under greenhouse conditions. Other hormone pathways are not strongly affected indicating ET signaling is only moderately activated,Citation3 which could facilitate a quicker launch of SA responses (“priming”). Increasing genetic variation via CRISPR/Cas-mediated genome editing is therefore a promising approach to create new traits in crops. The deployment of functional genomics for the identification of novel target genes in resistance breeding is a pre-requisite to develop hypotheses which could be tested by genome editing via CRISPR/Cas. Such mutations are valuable resources to conduct further basic research in order to understand the molecular function of genes involved in the plant-pathogen interaction and resistance breeding. However, success in the greenhouse is not the final step in the breeding process and requires subsequent testing under field conditions, which is currently impossible in the EU.Citation40 In light of climate change it is urgently required to change the legal status of genome edited mutant plants to complete the breeding process and develop new crop varieties which meet all requirements, for the consumer, the environment and the farmers.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Hu D, Zhang W, Zhang Y, Chang S, Chen L, Chen Y, Shi Y, Shen J, Meng J, Zou J. Reconstituting the genome of a young allopolyploid crop, Brassica napus, with its related species. Plant Biotechnol J. 2019;17(6):1–4. doi:10.1111/pbi.13041.
- Depotter JR, Deketelaere S, Inderbitzin P, von Tiedemann A, Höfte M, Subbarao KV, Wood TA, Thomma BP. Verticillium longisporum, the invisible threat to oilseed rape and other Brassicaceous plant hosts. Mol Plant Pathol. 2016;17:1004–1016. doi:10.1111/mpp.12350.
- Pröbsting M, Schenke D, Hossain R, Häder C, Thurau T, Wighardt L, Schuster A, Zhou Z, Ye W, Rietz S, et al. Loss-of-function of CRT1a (Calreticulin) reduces susceptibility to Verticillium longisporum in both Arabidopsis thaliana and oilseed rape (Brassica napus). Plant Biotechnol J. 2020. [Epub ahead of print]. doi:10.1111/pbi.13394.
- Behrens FH, Schenke D, Hossain R, Cai D, Zhao Y, Zhu W, Cai D, Zhao Y, Ladewig L, Zhu W. Suppression of abscisic acid biosynthesis at the early infection stage of Verticillium longisporum in oilseed rape (Brassica napus). Mol Plant Pathol. 2019;20:1645–1661. doi:10.1111/mpp.12867.
- Lorenzo O, Chico JM, Sánchez-Serrano JJ, Solano R. JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell. 2004;16:1938–1950. doi:10.1105/tpc.022319.
- Yasuda M, Ishikawa A, Jikumaru Y, Seki M, Umezawa T, Asami T, Maruyama-Nakashita A, Kudo T, Shinozaki K, Yoshida S, et al. Antagonistic interaction between systemic acquired resistance and the abscisic acid-mediated abiotic stress response in Arabidopsis. Plant Cell. 2008;20(6):1678–1692. doi:10.1105/tpc.107.054296.
- Fan J, Hill L, Crooks C, Doerner P, Lamb C. Abscisic acid has a key role in modulating diverse plant-pathogen interactions. Plant Physiol. 2009;150(4):1750–1761. doi:10.1104/pp.109.137943.
- Cao FY, Yoshioka K, Desveaux D. The roles of ABA in plant-pathogen interactions. J Plant Res. 2011;124:489–499. doi:10.1007/s10265-011-0409-y.
- Xu J, Audenaert K, Hofte M, De Vleesschauwer D. Abscisic acid promotes susceptibility to the rice leaf blight pathogen xanthomonas oryzae pv oryzae by suppressing salicylic acid-mediated defenses. PLoS One. 2013;8(6):e67413. doi:10.1371/journal.pone.0067413.
- de Torres Zabala M, Bennett MH, Truman WH, Grant MR. Antagonism between salicylic and abscisic acid reflects early host-pathogen conflict and moulds plant defence responses. Plant J. 2009;59:375–386. doi:10.1111/j.1365-313X.2009.03875.x.
- Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazan K. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell. 2004;16(12):3460–3479. doi:10.1105/tpc.104.025833.
- Arc E, Sechet J, Corbineau F, Rajjou L, Marion-Poll A. ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination. Front Plant Sci. 2013;4:63.
- Iven T, König S, Singh S, Braus-Stromeyer SA, Bischoff M, Tietze LF, Braus GH, Lipka V, Feussner I, Dröge-Laser W. Transcriptional activation and production of tryptophan-derived secondary metabolites in arabidopsis roots contributes to the defense against the fungal vascular pathogen Verticillium longisporum. Mol Plant. 2012;5:1389–1402. doi:10.1093/mp/sss044.
- Roos J, Bejai S, Oide S, Dixelius C. RabGAP22 is required for defense to the vascular pathogen Verticillium longisporum and contributes to stomata immunity. PLoS One. 2014;9(2):e88187. doi:10.1371/journal.pone.0088187.
- Ralhan A, Schöttle S, Thurow C, Iven T, Feussner I, Polle A, Gatz C. The vascular pathogen Verticillium longisporum requires a jasmonic acid-independent COI1 function in roots to elicit disease symptoms in Arabidopsis shoots. Plant Physiol. 2012;159:1192–1203. doi:10.1104/pp.112.198598.
- Pieterse CM, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SC. Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol. 2012;28:489–521. doi:10.1146/annurev-cellbio-092910-154055.
- Mur LA, Prats E, Pierre S, Hall MA, Hebelstrup KH. Integrating nitric oxide into salicylic acid and jasmonic acid/ethylene plant defense pathways. Front Plant Sci. 2013;4:215. doi:10.3389/fpls.2013.00215.
- Li J, Brader G, Palva ET. The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell. 2004;16(2):319–331. doi:10.1105/tpc.016980.
- Wang J, Tao F, An F, Zou Y, Tian W, Chen X, Xu X, Hu X. Wheat transcription factor TaWRKY70 is positively involved in high-temperature seedling plant resistance to Puccinia striiformis f. sp. tritici. Mol Plant Pathol. 2017;18:649–661. doi:10.1111/mpp.12425.
- Xiong XP, Sun SC, Zhang XY, Li YJ, Liu F, Zhu QH, Xue F, Sun J. GhWRKY70D13 regulates resistance to verticillium dahliae in cotton through the ethylene and jasmonic acid signaling pathways. Front Plant Sci. 2020;11:69. doi:10.3389/fpls.2020.00069.
- Fröschel C, Iven T, Walper E, Bachmann V, Weiste C, Dröge-Laser W. A gain-of-function screen reveals redundant ERF transcription factors providing opportunities for resistance breeding toward the vascular fungal pathogen Verticillium longisporum. Mol Plant Microbe Interact. 2019;32(9):1095–1109. doi:10.1094/MPMI-02-19-0055-R.
- Huang PY, Catinot J, Zimmerli L. Ethylene response factors in Arabidopsis immunity. J Exp Bot. 2016;67(5):1231–1241. doi:10.1093/jxb/erv518.
- Johansson A, Staal J, Dixelius C. Early responses in the Arabidopsis-Verticillium longisporum pathosystem are dependent on NDR1, JA- and ET-associated signals via cytosolic NPR1 and RFO1. Mol Plant Microbe Interact. 2006;19:958–969. doi:10.1094/MPMI-19-0958.
- Roos J, Bejai S, Mozūraitis R, Dixelius C. Susceptibility to Verticillium longisporum is linked to monoterpene production by TPS23/27 in Arabidopsis. Plant J. 2015;81(4):572–585. doi:10.1111/tpj.12752.
- Huang YF, Chen CT, Kao CH. Salicylic acid inhibits the biosynthesis of ethylene in detached rice leaves. Plant Growth Regul. 1993;12:79–82. doi:10.1007/BF00144586.
- Leslie CA, Romani RJ. Inhibition of ethylene biosynthesis by salicylic Acid. Plant Physiol. 1988;88(3):833–837. doi:10.1104/pp.88.3.833.
- Nazar R, Umar S, Khan NA, Sareer O. Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. S Afr J Bot. 2015;98:84–94. doi:10.1016/j.sajb.2015.02.005.
- Martinez C, Blanc F, Le Claire E, Besnard O, Nicole M, Baccou JC. Salicylic acid and ethylene pathways are differentially activated in melon cotyledons by active or heat-denatured cellulase from Trichoderma longibrachiatum. Plant Physiol. 2001;127(1):334–344. doi:10.1104/pp.127.1.334.
- Tirani MM, Nasibi F, Kalantari FHM. Interaction of salicylic acid and ethylene and their effects on some physiological and biochemical parameters in canola plants (Brassica napus L.). Photosynthetica. 2013;51:411–418. doi:10.1007/s11099-013-0041-2.
- Huang P, Dong Z, Guo P, Zhang X, Qiu Y, Li B, Wang Y, Guo H. Salicylic acid suppresses apical hook formation via NPR1-mediated repression of EIN3 and EIL1 in Arabidopsis. Plant Cell. 2020;32(3):612–629. doi:10.1105/tpc.19.00658.
- Chen H, Xue L, Chintamanani S, Germain H, Lin H, Cui H, Cai R, Zuo J, Tang X, Li X, et al. ETHYLENE INSENSITIVE3 and ETHYLENE INSENSITIVE3-LIKE1 repress SALICYLIC ACID INDUCTION DEFICIENT2 expression to negatively regulate plant innate immunity in Arabidopsis. Plant Cell. 2009;21(8):2527–2540. doi:10.1105/tpc.108.065193.
- Li Z, Liu H, Ding Z, Yan J, Yu H, Pan R, Hu J, Guan Y, Hua J. Low temperature enhances plant immunity via salicylic acid pathway genes that are repressed by ethylene. Plant Physiol. 2020;182(1):626–639. doi:10.1104/pp.19.01130.
- Shen X, Liu H, Yuan B, Li X, Xu C, Wang S. OsEDR1 negatively regulates rice bacterial resistance via activation of ethylene biosynthesis. Plant Cell Environ. 2011;34(2):179–191. doi:10.1111/j.1365-3040.2010.02219.x.
- Rao MV, Lee HI, Davis KR. Ozone-induced ethylene production is dependent on salicylic acid, and both salicylic acid and ethylene act in concert to regulate ozone-induced cell death. Plant J. 2002;32(4):447–456. doi:10.1046/j.1365-313X.2002.01434.x.
- Nissen P. Stimulation of somatic embryogenesis in carrot by ethylene; Effects of modulators of elhylene biosynthesis and action. Physiol Plant. 1994;92:397–403. doi:10.1111/j.1399-3054.1994.tb08827.x.
- O’Donnell PJ, Jones JB, Antoine FR, Ciardi J, Klee HJ. Ethylene-dependent salicylic acid regulates an expanded cell death response to a plant pathogen. Plant J. 2001;25(3):315–323. doi:10.1046/j.1365-313x.2001.00968.x.
- Schenke D, Naito K, Toyoda K, Shiraishi T, Inagaki Y, Ichinose Y. Regulation of elicitin-induced ethylene production in suspension cultured tobacco BY-2 cells. J Gen Plant Pathol. 2005;71(4):273–279. doi:10.1007/s10327-005-0197-0.
- Zheng X, Koopmann B, von Tiedemann A. Role of salicylic acid and components of the phenylpropanoid pathway in basal and cultivar-related resistance of oilseed rape (Brassica napus) to Verticillium longisporum. Plants (Basel). 2019;8(11):491.
- Robison MM, Griffith M, Pauls KP, Glick BR. Dual role for ethylene in susceptibility of tomato to Verticillium wilt. J Phytopathol. 2001;149(7–8):385–388. doi:10.1046/j.1439-0434.2001.00639.x.
- Urnov FD, Ronald PC, Carroll D. A call for science-based review of the European court’s decision on gene-edited crops. Nat Biotechnol. 2018;36(9):800–802. doi:10.1038/nbt.4252.