ABSTRACT
Hydrogen sulfide (H2S) is an important gas signal molecule, but little is known about its signal mechanism. Ca2+ is an important second messenger in plant cells, and its fluctuation in the cytoplasm causes downstream physiological responses. Our previous study found that H2S can induce the accumulation of hydrogen peroxide (H2O2). We also found that reactive oxygen species (ROS) can further induce the Ca2+ influx in guard cells by noninvasive micro-teat technology (NMT). This study confirmed that the accumulation of reactive oxygen species to induce Ca2+ signal in guard cells, resulting in stomatal closure. Thus, revealing a novel mechanism of H2S promoting stomatal closure.
KEYWORDS:
H2S is an important gas signal molecule. In plants, there are mainly three kinds of gas signal molecules: Nitric oxide (NO), Carbon monoxide (CO) and H2S.Citation1 H2S is involved in regulating stomatal movement, promoting root development, affecting cytoskeleton, alleviating drought and metal ions.Citation2–6 Although many studies have been done on the physiological effects of H2S, little is known about its detailed mechanism. At present, based on the chemical properties of H2S, two regulating mechanisms have been proposed. First, H2S can redox with ROS, H2O2 and peroxynitrite to reduce cellular oxidation level;Citation1 Second, H2S can mediate post-translational modification on the cysteine residue of the protein, namely persulfidation.Citation7
Ca2+ is an important second messenger in plant cells and plays a key role in regulating plant growth and development and responding to stress.Citation8 The most important calcium ion pool in plant cells is vacuoles, and calcium ions also exist in chloroplasts, mitochondria and endoplasmic reticulum. There are voltage-gated channels on the vacuole membrane. When cells are stimulated, calcium ions in the calcium pool flow into the cytoplasm and stimulate downstream signal molecules.Citation9 As an important signal molecule in cells, calcium ions have fingerprint characteristics, and their fluctuation is an important mechanism for maintaining physiological and biochemical functions in organisms. The volatility of calcium ions is highly consistent at the spatial and practical levels, which is characterized by different frequencies, periods or amplitudes.Citation10 Calmodulin is a receptor protein in calcium signal transduction, and its main function is to regulate the expression of downstream proteins and the physiological process of guard cells.Citation8
Our previous report showed that H2S could induce ROS accumulation in Arabidopsis thaliana roots.Citation11 In this study, H2O2 was visualized using the specific H2O2 fluorescent probe dichlorofluorescein diacetate (H2DCF-DA) according to the method described by Maffei et al.Citation12 We found that H2S could induce the accumulation of H2O2 in guard cells of leaf in Arabidopsis thaliana (,b). Recent studies have also shown that H2S activates NADPH oxidase RBOHD and RBOHF through S-thiosulfhydrylation, to induce H2O2 accumulation.Citation14 Reactive oxygen species can further induce the appearance of Ca2+ signal.Citation8 In this study, net stomatal fluxes of Ca2+ were measured with a noninvasive micro-test technology (NMT), according to methods described by Chen et al.Citation13 and Yan et al.Citation15 The epidermal strips were peeled from the rosette leaves of 5-week-old seed. A 100 mM NaHS or or 10 mM H2O2 was added instantly, after 3–5 min of detecting the basic Ca2+ flux. Data were recorded for 10 min. As shown in , the application of 100 μM NaHS and 10 mM H2O2 caused a significant Ca2+ influx in guard (approximately 63.6 ± 11 and 32.3 ± 7 pmol cm−2 s−1, respectively). Therefore, we believe that H2S can promote the accumulation of ROS and induce the emergence of Ca2+ signal in plant guard cells.
Figure 1. Assay of H2O2 content and net Ca2+ fluxes in Arabidopsis thaliana guard cells. (a) The effect of NaHS on H2O2 content in guard cells. 100 μM NaHS were used for various treatments for 20 min. Bar = 10 μm. (b) Quantification of H2O2 H2DCF-DA fluorescence density for (a). The average of the fluorescence of each guard cell was calculated. The data are the mean values ± SE (n = 10). (c) The effect of NaHS and H2O2 on Ca2+ fluxes in guard cells. The Ca2+ influxes in guard cells were measured, and 100 mM NaHS or 10 mM H2O2 was used. The negative value indicates influx. The data are the mean values ± SE (n = 3). (d) H2S induces Ca2+ signal in guard cells by regulating protein persulfidation and reactive oxygen species accumulation.Citation13,Citation14 Within each set of experiments, bars with different letters are significantly different at the P < .05 level (Duncan’s multiple range tests)
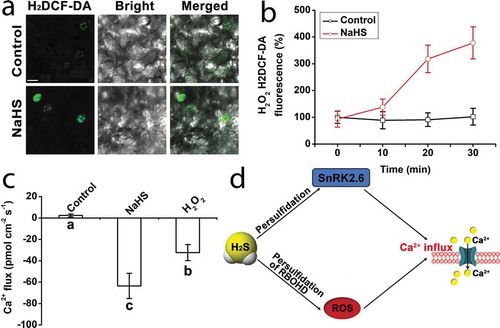
In our recent report, it has been proved that H2S can also activate ABA-SnRK2.6 signal pathway to induce the emergence of Ca2+ signal in guard cells.Citation13 In persulfidation-site mutant lines, persulfidation of SnRK2.6 resulted in a decrease of Ca2+ influx induced by ABA and H2S (). H2S also can activate NADPH oxidase RBOHD and RBOHFCitation14 (). Both H2S and H2O2 induce the stomatal closure, in which H2S activates NADPH oxidase RBOH and promotes the accumulation of H2O2.Citation13,Citation14 H2S does not induce Ca2+ influx in ost1-3, suggesting that H2S induces Ca2+ influx through persulfidation of SnRK2.6. The increase of Ca2+ leads to stomatal closure by inhibiting inward K+ channels and activating the outward anion channels.Citation8 As we know K+ and anion result in a dramatic decrease in osmotic pressure. As a result, a large amount of water flux from guard cells following stomatal closure. However, the increase of Ca2+ content results in partial or complete loss of stomatal closure in persulfidation-site mutant lines. And drought intolerance occurred in persulfidation-site mutants, especially in ost1-3/2.6 C131SC137S-GFP double-site mutant lines.Citation13
In conclusion, H2S can induce Ca2+ signal in guard cells by regulating the persulfidation of SnRK2.6 protein kinase and the accumulation of reactive oxygen speciesCitation13 (). This study revealed a novel mechanism that H2S promotes stomatal closure.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Corpas FJ, Gonzá lez-Gordo S, Cañas A, Palma JM. Nitric oxide and hydrogen sulfide in plants: which comes first? J Exp Bot. 2019;70:1–3. doi:https://doi.org/10.1093/jxb/erz031.
- Jia H, Chen S, Li D, Liesche J, Si C, Wang J, Ren M, Wang X, Yang J, Shi W, et al. Ethylene-induced hydrogen sulfide negatively regulates ethylene biosynthesis by persulfidation of ACO in tomato under osmotic stress. Front Plant Sci. 2018a;9:1517. doi:https://doi.org/10.3389/fpls.2018.01517.
- Jia H, Chen S, Wang X, Shi C, Liu K, Zhang S, Li J. Copper oxide nanoparticles alter cellular morphology via disturbing the actin cytoskeleton dynamics in Arabidopsis roots. Nanotoxicology. 2020;5:1–18. doi:https://doi.org/10.1080/17435390.2019.1678693.
- Jia H, Yang J, Liu H, Liu K, Ma P, Chen S, Shi W, Wei T, Ren X, Guo J, et al. Hydrogen sulfide - cysteine cycle plays a positive role in Arabidopsis responses to Copper Oxide nanoparticles stress. Environ Exp Bot. 2018b;155:195–205. doi:https://doi.org/10.1016/j.envexpbot.2018.06.034.
- Li J, Chen S, Wang X, Shi C, Liu H, Yang J, Shi W, Guo J, Jia H. Hydrogen sulfide disturbs actin polymerization via S-sulfhydration resulting in stunted root hair growth. Plant Physiol. 2018;178:936–949. doi:https://doi.org/10.1104/pp.18.00838.
- Xie Y, Mao Y, Zhang W, Lai D, Wang Q, Shen W. Reactive oxygen species-dependent Nitric oxide production contributes to hydrogen-promoted stomatal closure in Arabidopsis. Plant Physiol. 2014;165:759-773. doi: https://doi.org/10.1104/pp.114.237925.
- Aroca Á, Benito JM, Gotor C, Romero LC. Persulfidation proteome reveals the regulation of protein function by hydrogen sulfide in diverse biological processes in Arabidopsis. J Exp Bot. 2017;68:4915–4927. doi:https://doi.org/10.1093/jxb/erx294.
- Kim T, Böhmer M, Hu H, Nishimura N, Schroeder J. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol. 2010;61:561–591. doi:https://doi.org/10.1146/annurev-arplant-042809-112226.
- Gilroy S, Read N, Trewavas A. Elevation of cytoplasmic calcium by caged calcium or caged inositol trisphosphate initiates stomatal closure. Nature. 1990;346:769–771. doi:https://doi.org/10.1038/346769a0.
- Allen G, Chu S, Harrington C, Schumacher K, Hoffmann T, Tang Y, Grill E, Schroeder J. A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature. 2001;411:1053-1057. doi: https://doi.org/10.1038/35082575
- Li J, Jia H, Wang J, Cao Q, Wen Z. Hydrogen sulfide is involved in maintaining ion homeostasis via regulating plasma membrane Na+/H+antiporter system in the hydrogen peroxide-dependent manner in salt-stress Arabidopsis thaliana root. Protoplasma. 2014;251:899–912. doi: https://doi.org/10.1007/s00709-013-0592-x.
- Maffei M, Mithofer A, Arimura G. Effects of feeding Spodoptera littoralis on Lima bean leaves: III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol. 2006;140:1022–1035. doi:https://doi.org/10.1104/pp.105.071993.
- Chen SS, Jia HL, Wang XF, Shi C, Wang X, Ma P, Wang J, Wang MJ, Li JS. Hydrogen sulfide positively regulates abscisic acid signaling through persulfidation of SnRK2.6 in guard cells. Mol Plant. 2020;13:732–744. doi:https://doi.org/10.1016/j.molp.2020.01.004.
- Shen J, Zhang J, Zhou M, Zhou H, Cui B, Gotor C, Romero LC, Fu L, Yang J, Foyer CH, et al. Persulfidation-based modification of cysteine desulfhydrase and the NADPH oxidase RBOHD controls guard cell abscisic acid signaling. Plant Cell. 2020;32:1000–1017. doi:https://doi.org/10.1105/tpc.19.00826.
- Yan SL, Luo ST, Dong SS, Zhang T, Sun JR, Wang NN, Yao HJ, Shen YB. Heterotrimeric G-proteins involved in the MeJA regulated ion flux and stomatal closure in Arabidopsis thaliana. Funct Plant Biol. 2015;42:126–135. doi:https://doi.org/10.1071/FP14162.
